Generating Intrafusal Skeletal Muscle Fibres in Vitro
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microanatomy of Muscles
Microanatomy of Muscles Anatomy & Physiology Class Three Main Muscle Types Objectives: By the end of this presentation you will have the information to: 1. Describe the 3 main types of muscles. 2. Detail the functions of the muscle system. 3. Correctly label the parts of a myocyte (muscle cell) 4. Identify the levels of organization in a skeletal muscle from organ to myosin. 5. Explain how a muscle contracts utilizing the correct terminology of the sliding filament theory. 6. Contrast and compare cardiac and smooth muscle with skeletal muscle. Major Functions: Muscle System 1. Moving the skeletal system and posture. 2. Passing food through the digestive system & constriction of other internal organs. 3. Production of body heat. 4. Pumping the blood throughout the body. 5. Communication - writing and verbal Specialized Cells (Myocytes) ~ Contractile Cells Can shorten along one or more planes because of specialized cell membrane (sarcolemma) and specialized cytoskeleton. Specialized Structures found in Myocytes Sarcolemma: The cell membrane of a muscle cell Transverse tubule: a tubular invagination of the sarcolemma of skeletal or cardiac muscle fibers that surrounds myofibrils; involved in transmitting the action potential from the sarcolemma to the interior of the myofibril. Sarcoplasmic Reticulum: The special type of smooth endoplasmic Myofibrils: reticulum found in smooth and a contractile fibril of skeletal muscle, composed striated muscle fibers whose function mainly of actin and myosin is to store and release calcium ions. Multiple Nuclei (skeletal) & many mitochondria Skeletal Muscle - Microscopic Anatomy A whole skeletal muscle (such as the biceps brachii) is considered an organ of the muscular system. Each organ consists of skeletal muscle tissue, connective tissue, nerve tissue, and blood or vascular tissue. -

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -

Characterization of Cecal Smooth Muscle Contraction in Laying Hens
veterinary sciences Communication Characterization of Cecal Smooth Muscle Contraction in Laying Hens Katrin Röhm 1, Martin Diener 2 , Korinna Huber 1 and Jana Seifert 1,* 1 Institute of Animal Science, University of Hohenheim, 70593 Stuttgart, Germany; [email protected] (K.R.); [email protected] (K.H.) 2 Institute of Veterinary Physiology and Biochemistry, Justus-Liebig University, 35392 Gießen, Germany; [email protected] * Correspondence: [email protected] Abstract: The ceca play an important role in the physiology of the gastrointestinal tract in chickens. Nevertheless, there is a gap of knowledge regarding the functionality of the ceca in poultry, especially with respect to physiological cecal smooth muscle contraction. The aim of the current study is the ex vivo characterization of cecal smooth muscle contraction in laying hens. Muscle strips of circular cecal smooth muscle from eleven hens are prepared to investigate their contraction ex vivo. Contraction is detected using an isometric force transducer, determining its frequency, height and intensity. Spontaneous contraction of the chicken cecal smooth muscle and the influence of buffers (calcium-free buffer and potassium-enriched buffer) and drugs (carbachol, nitroprusside, isoprenaline and Verapamil) affecting smooth muscle contraction at different levels are characterized. A decrease in smooth muscle contraction is observed when a calcium-free buffer is used. Carbachol causes an increase in smooth muscle contraction, whereas atropine inhibits contraction. Nitroprusside, isoprenaline and Verapamil result in a depression of smooth muscle contraction. In conclusion, the present results confirm a similar contraction behavior of cecal smooth muscles in laying hens as Citation: Röhm, K.; Diener, M.; shown previously in other species. -

Desmin- Cytoskeleton Marker Cat#: ET1606-30
rev. 04/13/16 Desmin- Cytoskeleton Marker Cat#: ET1606-30 Prod uct Type: R ecombinant r abbit mono clonal IgG, primary antibodies Species reactivity: Human, Mouse, Rat, Zebra fish Applications: WB, ICC/IF, IHC, FC Molecular Wt.: 53 kDa Description: Cytoskeletal intermediate filaments (IFs) constitute a diverse group of proteins that are expressed in a highly tissue - specific manner. IFs are constructed from two - chain α -helical coiled-coil molecules arranged on an imperfect helical Fig1: Western blot analysis of Desmin on lattice, and have been widely used as markers for distinguishing different lysates using anti-Desmin antibody at individual cell types within a tissue and identifying the origins 1/1,000 dilution. of metastatic tumors. Vimentin is an IF general marker of cells Positive control: originating in the mesenchyme. Vimentin and Desmin, a related class III IF, are both expressed during skeletal muscle development. Lane 1: Human skeletal muscle Desmin, a 469 amino acid protein found near the Z line in sarcomeres, Lane 2: Mouse heart is expressed more frequently in adult differentiated state tissues. Desmin makes up attachments between the terminal Z-disc and membrane-associated proteins to form a force-transmitting system. Mutations in the gene encoding for Desmin are associated with adult-onset skeletal myopathy, sporadic disease and mild cardiac involvement. Immunogen: Recombinant protein. Positive control: Fig2: ICC staining Desmin in C2C12 cells (green). C2C12, human uterus tissue, mouse bladder tissue, mouse heart The nuclear counter stain is DAPI (blue). Cells tissue, mouse skeletal muscle tissue. were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS. -

The Muscular System
THE MUSCULAR SYSTEM COMPILED BY HOWIE BAUM 1 Muscles make up the bulk of the body and account for 1/3 of its weight.!! Blood vessels and nerves run to every muscle, helping control and regulate each muscle’s function. The muscular system creates body heat and also moves the: Bones of the Skeletal system Food through Digestive system Blood through the Circulatory system Fluids through the Excretory system MUSCLE TISSUE The body has 3 main types of muscle tissue 1) Skeletal, 2) Smooth, and 3) Cardiac SKELETAL MUSCLE SMOOTH MUSCLE CARDIAC MUSCLE Skeletal muscles attach to and move bones by contracting and relaxing in response to voluntary messages from the nervous system. Skeletal muscle tissue is composed of long cells called muscle fibers that have a striated appearance. Muscle fibers are organized into bundles supplied by blood vessels and innervated by motor neurons. Muscle structure Skeletal (striated or voluntary) muscle consists of densely packed groups of hugely elongated cells known as myofibers. These are grouped into bundles (fascicles). A typical myofiber is 2–3 centimeters ( 3/4–1 1/5 in) long and 0.05millimeters (1/500 inch) in diameter and is composed of narrower structures – myofibrils. These contain thick and thin myofilaments made up mainly of the proteins actin and myosin. Numerous capillaries keep the muscle supplied with the oxygen and glucose needed to fuel contraction. Skeletal Muscles • Skeletal muscles attach to bones by tendons (connective tissue) and enable movement. • Skeletal muscles are mostly voluntary Feel the back of your ankle to feel your Achilles tendon - the largest tendon in your body. -

Muscle Histology
Muscle Histology Dr. Heba Kalbouneh Functions of muscle tissue ▪ Movement ▪ Maintenance of posture ▪ Joint stabilization ▪ Heat generation Types of Muscle Tissue ▪ Skeletal muscle ▪ Cardiac muscle ▪ Smooth muscle Types of Muscle Tissue Skeletal •Attach to and move skeleton •40% of body weight •Fibers = multinucleate cells (embryonic cells fuse) •Cells with obvious striations •Contractions are voluntary Cardiac: only in the wall of the heart •Cells are striated •Contractions are involuntary (not voluntary) Smooth: walls of hollow organs •Lack striations •Contractions are involuntary (not voluntary) Similarities… ▪ Their cells are called fibers because they are elongated ▪ Contraction depends on myofilaments ▪ Actin ▪ Myosin ▪ Plasma membrane is called sarcolemma ▪ Sarcos = flesh ▪ Lemma = sheath SKELETAL MUSCLES Epimysium: surrounds whole muscle Endomysium is around each muscle fiber Perimysium is around fascicle = muscle cell= myofiber Skeletal muscle This big cylinder is a fiber: a cell ▪ Fibers (each is one cell) have striations ▪ Myofibrils are organelles of the cell: these are made -an organelle up of myofilaments ▪ Sarcomere ▪ Basic unit of contraction ▪ Myofibrils are long rows of repeating sarcomeres ▪ Boundaries: Z discs (or lines) Sarcomere M line provides an attachment to myosin filaments Z line provides an attachment to actin filaments A band is the darker band of the myofibril containing myosin filaments H band is the lighter section in the middle of the A band where only myosin is present I band is the lighter band containing -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
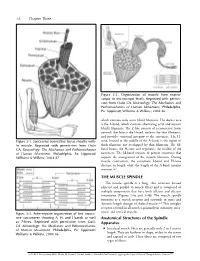
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

The Role of the Actin Cytoskeleton During Muscle Development In
THE ROLE OF THE ACTIN CYTOSKELETON DURING MUSCLE DEVELOPMENT IN DROSOPHILA AND MOUSE by Shannon Faye Yu A Dissertation Presented to the Faculty of the Louis V. Gerstner, Jr. Graduate School of the Biomedical Sciences in Partial Fulfillment of the Requirements of the Degree of Doctor of Philosophy New York, NY Oct, 2013 Mary K. Baylies, PhD! Date Dissertation Mentor Copyright by Shannon F. Yu 2013 ABSTRACT The actin cytoskeleton is essential for many processes within a developing organism. Unsurprisingly, actin and its regulators underpin many of the critical steps in the formation and function of muscle tissue. These include cell division during the specification of muscle progenitors, myoblast fusion, muscle elongation and attachment, and muscle maturation, including sarcomere assembly. Analysis in Drosophila has focused on regulators of actin polymerization particularly during myoblast fusion, and the conservation of many of the actin regulators required for muscle development has not yet been tested. In addition, dynamic actin processes also require the depolymerization of existing actin fibers to replenish the pool of actin monomers available for polymerization. Despite this, the role of actin depolymerization has not been described in depth in Drosophila or mammalian muscle development. ! Here, we first examine the role of the actin depolymerization factor Twinstar (Tsr) in muscle development in Drosophila. We show that Twinstar, the sole Drosophila member of the ADF/cofilin family of actin depolymerization proteins, is expressed in muscle where it is essential for development. tsr mutant embryos displayed a number of muscle defects, including muscle loss and muscle misattachment. Further, regulators of Tsr, including a Tsr-inactivating kinase, Center divider, a Tsr-activating phosphatase, Slingshot and a synergistic partner in depolymerization, Flare, are also required for embryonic muscle development. -

Muscle Physiology Dr
Muscle Physiology Dr. Ebneshahidi Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Skeletal Muscle Figure 9.2 (a) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Functions of the muscular system . 1. Locomotion . 2. Vasoconstriction and vasodilatation- constriction and dilation of blood vessel Walls are the results of smooth muscle contraction. 3. Peristalsis – wavelike motion along the digestive tract is produced by the Smooth muscle. 4. Cardiac motion . 5. Posture maintenance- contraction of skeletal muscles maintains body posture and muscle tone. 6. Heat generation – about 75% of ATP energy used in muscle contraction is released as heat. Copyright. © 2004 Pearson Education, Inc., publishing as Benjamin Cummings . Striation: only present in skeletal and cardiac muscles. Absent in smooth muscle. Nucleus: smooth and cardiac muscles are uninculcated (one nucleus per cell), skeletal muscle is multinucleated (several nuclei per cell ). Transverse tubule ( T tubule ): well developed in skeletal and cardiac muscles to transport calcium. Absent in smooth muscle. Intercalated disk: specialized intercellular junction that only occurs in cardiac muscle. Control: skeletal muscle is always under voluntary control‚ with some exceptions ( the tongue and pili arrector muscles in the dermis). smooth and cardiac muscles are under involuntary control. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Innervation: motor unit . a) a motor nerve and a myofibril from a neuromuscular junction where gap (called synapse) occurs between the two structures. at the end of motor nerve‚ neurotransmitter (i.e. acetylcholine) is stored in synaptic vesicles which will release the neurotransmitter using exocytosis upon the stimulation of a nerve impulse. Across the synapse the surface the of myofibril contains receptors that can bind with the neurotransmitter. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body.