Ferrous Friction Stir Weld Physical Simulation A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parshwamani Metals
+91-8048554624 Parshwamani Metals https://www.indiamart.com/parshwamanimetals/ Parshwamani Metals is one of the leading manufacturers, supplier and traders of Industrial Metal Tube, Beryllium Product, Shim Sheet, SS Round And Square Bar, Aluminium Products, Aluminum Bronze Products etc. About Us Parshwamani Metals was established in the year 2015 as a professionally managed Manufacturer, Trader and Wholesaler specialized in providing premium grade Copper and Brass Metals Products. Today, we endeavor to revolutionize the industry by fabricating a wide gamut of quality products, which includes Brass Products, Copper Products and Copper Alloy. Our claim to success is hallmarked by the offered quality products that gained us huge recognizance for its high strength, wear and tear resistance, accurate dimensions, flexibility and durable finish. Our products find their wide applications in architectural fittings, hardware and telecommunication. Owing to swift delivery schedules, easy payment modes and overt business practices, we have been successful in earning huge client base. We deal in Jindal Brand. Our efforts are determined with the objective of industrial leadership that equips our team members to manufacture customized products. And, to achieve this, we have developed modernized R&D centers and cutting edge manufacturing facilities. Furthermore, the facility is divided into various functional units like procurement, engineering, production, research & development, quality-testing, warehousing & packaging etc. Our organization is backed -

Planning for Seafood Freezing
TTTTTTTTTTT Planning for Seafood Freezing Edward KOLBE Donald KRAMER MAB-60 2007 Alaska Sea Grant College Program University of Alaska Fairbanks Fairbanks, Alaska 99775-5040 (888) 789-0090 Fax (907) 474-6285 www.alaskaseagrant.org TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT Elmer E. Rasmuson Library Cataloging-in-Publication Data: Kolbe, Edward. Planning for seafood freezing ⁄ Edward Kolbe and Donald Kramer. – Fairbanks, Alaska : Alaska Sea Grant College Program, University of Alaska Fairbanks, 2007 126 p. : 51 ill. ; cm. (Alaska Sea Grant College Program, University of Alaska Fairbanks ; MAB-60) Includes bibliographical references and index. 1. Frozen seafood—Preservation—Handbooks, manuals, etc. 2. Seafood— Preservation—Handbooks, manuals, etc. 3. Cold storage—Planning—Handbooks, manuals, etc. 4. Fishery management—Handbooks, manuals, etc. 5. Refrigeration and refrigeration machinery—Handbooks, manuals, etc. 6. Frozen fishery products—Handbooks, manuals, etc. I. Title. II. Kramer, Donald E. III. Series: Alaska Sea Grant College Program ; MAB-60. SH336.F7 K65 2007 ISBN 1-56612-119-1 Credits The work for this book was funded in part by the NOAA Office of Sea Grant, U.S. Department of Commerce, under grants NA76RG0476 (OSU), NA86RG0050 (UAF), and NA76RG0119 (UW); projects A/ESG-3 (OSU), A/151-01 (UAF), and A/FP-7 (UW), and by appropriations made by the Oregon, Alaska, and Washington state legislatures. Publishing is supported by grant NA06OAR4170013, project A/161-01. Sea Grant is a unique partnership with public and private sectors, combining research, education, and technology transfer for public service. This national network of universities meets the changing environmental and economic needs of people in our coastal, ocean, and Great Lakes regions. -

Specifications of 2 Kelvin J-T Heat Exchanger
Specification of Brazed aluminium plate fin heat exchanger A. Scope of Supply (Qty-1 No.) Fabrication and supply of brazed aluminium plate fin heat exchanger as per parameters described in Table-1. B. Bidder Qualification Criteria 1. Original Manufacturer must have enough experience and knowledge of complete manufacturing cycle of brazed plate fin heat exchanger. He must have experience in supplying brazed plate fin heat exchanger. The supplier shall submit/upload supporting documents (scan copy of purchase order) for the same along with bid. 2. If the bidder is not the original manufacturer then he must submit the details of original manufacturer/fabricator. And quotation will be accepted only when the original manufacturer/fabricator qualify the above mentioned criteria. C. Design Details and Requirements 1. The manufacturer will prepare all the fabrication drawings according to the details mentioned in Table 1, with necessary flow distributor,headers and nozzles for passage of low pressure and high pressure stream. It is manufacturer’s responsibility to prepare final fabrication drawings of complete unit with all necessary details and get written approval from purchaser before commencing the fabrication. 2. The heat exchanger shall be made as per the standard tolerances given in ALPEMA code for the external dimensions of brazed Aluminium plate-fin heat exchangers. Table 1. Details of various parameters of plate fin heat exchanger Parameters Details Working Fluid (hot and cold side) Helium Heat Exchanger core Material ASTM 3003 Aluminium alloy Headers and Nozzle Material ASTM 5083/5454 Aluminium alloy Mass flow rate 6 g/s Operating pressure 6 bara maximum for hot side 16 mbara for cold side Fin type Offset Serrated Fin Fin density 14 fins/inch (551 fins/m) +0.2mm Fin height for hot side 3.8mm−0mm Fin height for cold side +0.2mm 6.5mm−0mm Fin Flow length Minimum 3mm and Maximum 5 mm Fin Thickness 0.2 mm Parting sheet thickness 0.8 mm Side bar thickness 8 mm No. -

Machining of Aluminum and Aluminum Alloys / 763
ASM Handbook, Volume 16: Machining Copyright © 1989 ASM International® ASM Handbook Committee, p 761-804 All rights reserved. DOI: 10.1361/asmhba0002184 www.asminternational.org MachJning of Aluminum and AlumJnum Alloys ALUMINUM ALLOYS can be ma- -r.. _ . lul Tools with small rake angles can normally chined rapidly and economically. Because be used with little danger of burring the part ," ,' ,,'7.,','_ ' , '~: £,~ " ~ ! f / "' " of their complex metallurgical structure, or of developing buildup on the cutting their machining characteristics are superior ,, A edges of tools. Alloys having silicon as the to those of pure aluminum. major alloying element require tools with The microconstituents present in alumi- larger rake angles, and they are more eco- num alloys have important effects on ma- nomically machined at lower speeds and chining characteristics. Nonabrasive con- feeds. stituents have a beneficial effect, and ,o IIR Wrought Alloys. Most wrought alumi- insoluble abrasive constituents exert a det- num alloys have excellent machining char- rimental effect on tool life and surface qual- acteristics; several are well suited to multi- ity. Constituents that are insoluble but soft B pie-operation machining. A thorough and nonabrasive are beneficial because they e,,{' , understanding of tool designs and machin- assist in chip breakage; such constituents s,~ ,.t ing practices is essential for full utilization are purposely added in formulating high- of the free-machining qualities of aluminum strength free-cutting alloys for processing in alloys. high-speed automatic bar and chucking ma- Strain-hardenable alloys (including chines. " ~ ~p /"~ commercially pure aluminum) contain no In general, the softer ailoys~and, to a alloying elements that would render them lesser extent, some of the harder al- c • o c hardenable by solution heat treatment and ,p loys--are likely to form a built-up edge on precipitation, but they can be strengthened the cutting lip of the tool. -

Aluminium Alloys Chemical Composition Pdf
Aluminium alloys chemical composition pdf Continue Alloy in which aluminum is the predominant lye frame of aluminum welded aluminium alloy, manufactured in 1990. Aluminum alloys (or aluminium alloys; see spelling differences) are alloys in which aluminium (Al) is the predominant metal. Typical alloy elements are copper, magnesium, manganese, silicon, tin and zinc. There are two main classifications, namely casting alloys and forged alloys, both further subdivided into heat-treatable and heat-free categories. Approximately 85% of aluminium is used for forged products, e.g. laminated plates, foils and extrusions. Aluminum cast alloys produce cost-effective products due to their low melting point, although they generally have lower tensile strength than forged alloys. The most important cast aluminium alloy system is Al–Si, where high silicon levels (4.0–13%) contributes to giving good casting features. Aluminum alloys are widely used in engineering structures and components where a low weight or corrosion resistance is required. [1] Alloys composed mostly of aluminium have been very important in aerospace production since the introduction of metal leather aircraft. Aluminum-magnesium alloys are both lighter than other aluminium alloys and much less flammable than other alloys containing a very high percentage of magnesium. [2] Aluminum alloy surfaces will develop a white layer, protective of aluminum oxide, if not protected by proper anodization and/or dyeing procedures. In a wet environment, galvanic corrosion can occur when an aluminum alloy is placed in electrical contact with other metals with a more positive corrosion potential than aluminum, and an electrolyte is present that allows the exchange of ions. -

The Development of Process Technology for the Friction Stir Welding of Thick Section Aluminium Alloys
U ìo The Development of Process Technology for the Friction Stir Welding of Thick Section Aluminium Alloys Rudolf Zetller School of Mechanical Engineering Faculty of Engineering, Computer and Mathematical Sciences University of Adelaide July 2006 CONTENTS Abstract 4 Statement of Originality. 6 Acknowledgements ....... 7 1.1 Background... .8 1.2 Significance.. 10 1.3 Objectives..... 10 2. Literature Review ..,12 2.1 Classification For Wrought Aluminium Alloys........ .12 2.2 Strengthening Mechanisms ln Heat-Treatable Aluminium Alloys ....... .13 2.3 Strengthening Mechanisms I n Non-Heat-Treatable Aluminium Alloys 16 2.3.1 Annealing 17 2.3.2 Recovery 1B 2.3.3 Recrystallization 18 2.3.4 Grain Growth 20 2.3.5 Solid-Solution Strengthening 20 2.4 Friction Welding (FW): A Process Overview 21 2.5 FW: Weld Structure And Weld Formation 23 2.6 Fusion Welding Aluminium 30 2.7 Friction Stir Welding (FSW): A Process Overview.. 34 2.8 FSW: Weld Structure And Weld Formation 3B 2.9 The Origins Of The FSW Tool For Joining Aluminium And lts Alloys 46 2.10 FSW And Tool Rubbing Velocity Relationships........... 48 2.11 Limitations Found To Exist For The Original FSW Tool Design........ 53 2.12 Modifications To The Original FSW Tool Pin Design 55 2.13 Forces Encountered When FSW 69 2.14 FSW: Process Modelling With Reference To Flow Visualisation...... 72 2.15 Summary Of Key Points From The Literature Review............ B6 3. Materials, Plant And Equipment 88 3.1 Wrought Aluminum Alloys - Composition and Temper 88 3.2 lnvestigated Wrought Al Alloys - Properties............... -

A Review of Microstructural Changes Occurring During FSW in Aluminium Alloys and Their Modelling Dimitri Jacquin, Gildas Guillemot
A review of microstructural changes occurring during FSW in aluminium alloys and their modelling Dimitri Jacquin, Gildas Guillemot To cite this version: Dimitri Jacquin, Gildas Guillemot. A review of microstructural changes occurring during FSW in aluminium alloys and their modelling. Journal of Materials Processing Technology, Elsevier, 2021, 288, pp.116706. 10.1016/j.jmatprotec.2020.116706. hal-02911059 HAL Id: hal-02911059 https://hal.archives-ouvertes.fr/hal-02911059 Submitted on 3 Aug 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A review of microstructural changes occurring during FSW in aluminium alloys and their modelling Dimitri Jacquin a) Gildas Guillemot b) a) University of Bordeaux, I2M CNRS, Site IUT, 15, rue Naudet - CS 10207, 33175 Gradignan Cedex, France b) MINES ParisTech, PSL Research University, CEMEF UMR CNRS 7635, CS10207, 06904 Sophia Antipolis, France Abstract: Friction stir welding (FSW) process is currently considered as a promising alternative to join aluminium alloys. Indeed, this solid-state welding technique is particularly recommended for the assembly of these materials. Since parts are not heated above their melting temperature, FSW process may prevent solidification defects encountered in joining aluminium alloys and known as limitations to the dissemination of these materials in industries. -

Powder Metallurgy Review Autumn/Fall 2016 Vol 5 No 3
POWDER METALLURGY VOL. 5 NO. 3 VOL. 2016 AUTUMN/FALL REVIEW HOEGANAES INNOVATION CENTRE THE MASTER ALLOY ROUTE POWDERMET2016: REPORT & AWARDS Published by Inovar Communications Ltd www.pm-review.com PowderMetallurgy Review Summer 2016.pdf 1 4/24/16 10:42 PM FACILITIES PEOPLE PRODUCTS IMAGINATION DRIVES INNOVATION THE FUTURE OF PM SOLUTIONS HAS ARRIVED! The potential of powder metallurgy is only limited by one’s imagination… Hoeganaes Corporation, the world’s leader in the production of metal powders, has been the driving force behind the growth in the Powder Metallurgy industry for over 65 years. Hoeganaes has fueled that growth with successive waves of technology which have expanded the use of metal powders for a wide variety of applications. Hoeganaes Corporation production World Class site located in Bazhou City, Engineers and Hebei Province, China. Scientists at Hoeganaes’ new Innovation Center in the USA develop new products and processes that move the state of the industry forward, spanning Automotive applications to Additive Manufacturing. The Commencement of melting and atomization operations in Bazhou, China makes Hoeganaes Corporation the rst international-grade ferrous metal powder producer on the Chinese mainland. With production and mixing facilities across North America, Europe, and Asia, Hoeganaes serves our customers across the globe with the most advanced PM solutions. FOLLOW US ON SOCIAL MEDIA AT: MOBILE DEVICE APPS NOW AVAILABLE FOR DOWNLOAD @HoeganaesCorp © 2016 Hoeganaes Corporation Publisher & editorial offices Inovar -

Welding Journal {\SSH 0043-2296) Is Published R
w •' VII PUBLISHED BY THE AMERICAN WELDING SOCIETY TO ADVANCE THE SCIENCE, TECHNOLOGY, AND APPLICATION OF WELDING AND ALLIED JOINING AND CUTTING PROCESSES, INCLUDING BRAZING, SOLDERING, AND THERMAL SPRAYING One Word Select-Arc's New (Line and Reputati U-Si Qii^lily.r. 1 fk % Select-Arc, Inc. has earned teristics, consistent deposit electrodes' higher deposi- Discover for yourself the an outstanding reputation chemistry and excellent tion rates improve produc- many benefits of specifying in the industry as a manu- overall performance you tivity and reduce welding Select-Arc's new premium facturer of premium quality have come to expect from costs. stainless steel electrodes. Call tubular welding electrodes Select-Arc. us today at 1-800-341-3213 for carbon and low alloy SelectAlloy's smooth bead or you can visit our website steel welding. The chart below shows contour, easy peeling slag, at www.select-arc.com for that SelectAUoy flux cored minimal spatter, closely more information. Now Select-Arc has controlled weld deposit expanded its range of Typical Deposition Rates for compositions and metal exceptional products Various Stainless Steel Consumables soundness deliver addi- with the introduction tional savings. SELECT of a complete line of .0 45" Fll xCor .045" vletal ( lored Elect rode E ectrod The Select 400-Series austenitic, martensitic // metal cored electrodes and ferritic stainless / V sS r offer the same advan- steel electrodes. Both f s 600 Enterprise Drive the new SelectAUoy So 45" So IdWir e tages as SelectAUoy and ^ * Elect ode P.O. Box 259 and Select stainless are ideally suited for Fort Loramie,OH 45845-0259 steel wires deliver the difficult-to-weld appli- . -

NASA Technical Reports Server
00000001 r_,.............................................................................. -----T-____ 'I . .,,i.',',_.i IJ]//. /,-";:;E--,'_:;J 4 N_:CR'I12119-3:" '_ ,. ,, EVALUAT_:"'"::IONOF COATEDOoiuMBiui ALLOY ,_I',,"'Y>,L. • :_'.. ; " "_:i;,-', ... :_' '"._. :'.,: .,] • ." !--.':"' !;_ 'F . ' " "._'_ ,_., :,:- '. ". _ ' ,, .HEAT SHIELDSFOR/SPACESHUTTLE" THERMAL-PROTEC-Ti0NSYSTE- MA_PLiCATiON .m ;. .:/ VOLUMEIii " . 41. PH_EIil IGU_sizE_ _ALUATioN ,,mINIRA r JlDYNAMI..... CS:: ' ;" ,.>'," CorlWlir A!ace _vi_on " "_. ; (NASA-eR-i1211_.31 BVALU/LTZON OF COAi'ND COLU_IuH ALLOy HEAT SHIELDS FOR SgACZ N7g-35278 SHUTZLF THZE_AL PROTECTION SYSTEM APPLICATION. (General Dynamics/Convair) 1E2 p HC $11.25 Unclas ; CSCL 22B GJ/JI 51035 • Ll - :_, 00000001-TSA03 NASA_CB.-.l12119-3 EVALUATION OF COATED COLUMBIUM ALLOY HEAT SHIELDS FOR SPACE SHUTTLE TKERMAL PROTECTION SYSTEM APPLICATION VOLUME DI i J ! PHASE 11I - FULL SIZE TI:_ EVALUATION ":; March 1974 By .... J.W. BaVr and W. E. Black .. Prepared Under _,_ Contract NA81-9793 ,:, Prepared by .,' CONVAIR AEROSPACE DIVISIONOF G_ENERAL DYNA3_ICS .. ,: SanDiego,.CalleorMa for : : ,o: National Aeronauflos and Space Admi_efla_tlon ",i'".: LANGLEY RESEARCH CENTER Hampt_p virginia • - :.- _'-._- ....."_................ ::_-_7........_- "_.--_ ......i- ." _ . ... .."j....._.r".._ri:..:_"_-i;_2"'C.2_:'".'_,':_"-_- ',-_ 00000001-TSA04 FOREWORD ._lis report was prepared by Convair Aerospace Division of General Dynamics -- San Diego Operation under Contract NAS1-9793 for the National Aeron_tics and Space • Administration, Langley Research Center, Hampton, Virginia. R was administered under the direction of the Materials Division, Materiels Research Branch, with Mr. D. R. Rummier acting as the Technical l_presentative of the Contracting Officer. •: The Convair program manager and principal designer through Phase I and Phase II was Wo E. -
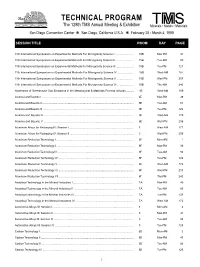
Technical Program
TECHNICAL PROGRAM The 128th TMS Annual Meeting & Exhibition T S The 128th TMS Annual Meeting & Exhibition Minerals • Metals • Materials San Diego Convention Center ✻ San Diego, California U.S.A. ✻ February 28 - March 4, 1999 SESSION TITLE ROOM DAY PAGE 11th International Symposium on Experimental Methods For Microgravity Science I ........................ 15B Mon-PM 37 11th International Symposium on Experimental Methods For Microgravity Science II ........................ 15B Tue-AM 80 11th International Symposium on Experimental Methods For Microgravity Science III ....................... 15B Tue-PM 121 11th International Symposium on Experimental Methods For Microgravity Science IV ...................... 15B Wed-AM 167 11th International Symposium on Experimental Methods For Microgravity Science V ...................... 15B Wed-PM 207 11th International Symposium on Experimental Methods For Microgravity Science VI ...................... 15B Thu-AM 240 Abatement of Greenhouse Gas Emissions in the Metallurgical & Materials Process Industry .......... 1B Wed-AM 169 Alumina and Bauxite I ......................................................................................................................... 6E Mon-PM 38 Alumina and Bauxite II ......................................................................................................................... 6E Tue-AM 81 Alumina and Bauxite III ........................................................................................................................ 6E Tue-PM 122 Alumina -

Welding and Inspection IV-6 4
LEGAL NOTICE This report was prepared solely for use (including release to the Public Document Room) by the U. S. Atomic Energy Commission in considering the application filed by Northern States Power Company (pursuant to the Atomic Energy Act of 1954, as amended, and Title 10 Part 50 of the Code of Federal Regulations) for a construction permit and operating license for a boiling water reactor nuclear generating plant to be located at Monticello, Minnesota. Use of this report or the information contained herein by any one other than the Atomic Energy Commission or for any purpose other than that for which it is expressly intended is not authorized. With respect to any unauthorized use neither Northern States Power Company, the General Electric Company, Chicago Bridge & Iron Company nor the individual authors: A. Makes any warranty or representation, expressed or implied, with respect to the accuracy, completeness, or usefulness of the information contained in this report, or that the use of any information disclosed in this report may not infringe privately owned rights; or B. Assumes any responsibility for liability or damage which may result from the use of any information disclosed in this report. A2A TABLE OF CONTENTS I. SUMMARY AND INTRODUCTION 1. 1 Summary I-1 1. 2 Study I-1 II. FEASIBILITY OF SHIPPING SHOP-ASSEMBLED VESSEL TO MONTICELLO SITE 2. 1 General H1-1 IIL REACTOR VESSEL DESIGN 3. 1 Design Requirements III-1 3. 2 Performance of Reactor Vessel Design Analysis 111-3 3. 3 Material Selection 111-6 3. 4 Appendix Material 111-6 IV.