Materials of Engineering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parshwamani Metals
+91-8048554624 Parshwamani Metals https://www.indiamart.com/parshwamanimetals/ Parshwamani Metals is one of the leading manufacturers, supplier and traders of Industrial Metal Tube, Beryllium Product, Shim Sheet, SS Round And Square Bar, Aluminium Products, Aluminum Bronze Products etc. About Us Parshwamani Metals was established in the year 2015 as a professionally managed Manufacturer, Trader and Wholesaler specialized in providing premium grade Copper and Brass Metals Products. Today, we endeavor to revolutionize the industry by fabricating a wide gamut of quality products, which includes Brass Products, Copper Products and Copper Alloy. Our claim to success is hallmarked by the offered quality products that gained us huge recognizance for its high strength, wear and tear resistance, accurate dimensions, flexibility and durable finish. Our products find their wide applications in architectural fittings, hardware and telecommunication. Owing to swift delivery schedules, easy payment modes and overt business practices, we have been successful in earning huge client base. We deal in Jindal Brand. Our efforts are determined with the objective of industrial leadership that equips our team members to manufacture customized products. And, to achieve this, we have developed modernized R&D centers and cutting edge manufacturing facilities. Furthermore, the facility is divided into various functional units like procurement, engineering, production, research & development, quality-testing, warehousing & packaging etc. Our organization is backed -
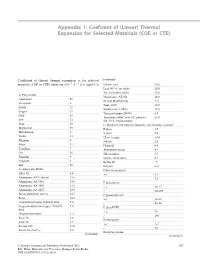
Linear) Thermal Expansion for Selected Materials (COE Or CTE
Appendix 1: Coefcient of (Linear) Thermal Expansion for Selected Materials (COE or CTE) Coefficient of (linear) thermal expansion, α, for selected (continued) − − materials (COE or CTE) (units are ×10 6 °C 1 (i.e. ppm/°C)) Indium–lead 33.0 Lead (95 %) tin solder 28.0 Tin–lead solder 60/40 25.0 A. Pure metals Magnesium, AZ31B 26.0 Aluminium 25 Ni-clad Molybdenum 5–6 Chromium 6 Steel, 1020 12.0 Cobalt 12 Stainless steel (18-8) 17.0 Copper 17 Tungsten/copper (90/10) 6.5 Gold 14 Aluminium MMC with SiC particles 6–14 Iron 12 (80–50 % reinforcement) Lead 29 C. Insulators and substrate materials (for electronic systems)a Magnesium 25 E glass 5.5 Molybdenum 5 S glass 2.6 Nickel 13 Glass–ceramic >3.0 Platinum 9 Silicon 2.6 Silver 19 Diamond 0.9 Tantalum 7 Aluminium nitride 4.5 Tin 20 Silicon nitride 3.7 Titanium 9 Quartz, fused silica 0.5 Tungsten 5 Kevlar 49 –5 Zinc 35 Beryllia 6–9 B. Alloys and MMCs Cubic boron nitride Alloy 42 4.4 x–y 3.7 Aluminium (40 % silicon) 13.5 z 7.2 Aluminium, AA 6061 23.6 E glass/epoxy Aluminium, AA 3003 23.2 x–y 14–17 Aluminium, AA 2017 22.9 z 80–280 Boron aluminium (20 %) 12.7 E glass/polyimide Brass 18.0 x–y 12–16 Copper/invar/copper 20/60/20 thick 5.8 z 40–80 Copper/molybdenum/copper 20/60/20 7.0 E glass/PTFE thick x–y 24 Graphite/aluminium 4–6 z 260 Invar 36 1.6 Kevlar/epoxy Invar 42 4.5 x–y 5–7 Inconel 600 13.0 z 70 Kovar (Fe–Ni–Co) 5.0 (continued) Kevlar/polyimide (continued) © Springer International Publishing Switzerland 2016 557 B.D. -

924 Iaea-Sm-310/ 69P
924 IAEA-SM-310/ 69P RESULTS FROM POST-MORTEM TESTS WITH MATERIAL FROM THE OLD CORE-BOX OF THE HIGH FLUX REACTOR (HFR) AT PETTEN M.I. de Vries Netherlands Energy Research Foundation, ECN, Eetten, The Netherlands M.R.. Cundy Joint Research Centre, Patten, The Netherlands 925 RESULTS FROM POST-MORTEM TESTS WITH MATERIAL FROM THE OLD CORE-BOX OF THE HIGH FLUX REACTOR (HFR) AT PETTEN ABSTRACT Results are reported from hardness measurements, tensile tests and fracture mechanics experiments (fatigue crack growth and fracture toughness) on 5154 aluminium specimens, fabricated from remnants of the old HFR core box. The specimen material was exposed to a maximum thermal neutron fluence of 7.5 * 1026n/m2(E < 0.4eV). Test results for this fluence (ratio of the thermal to fast neutron flux density is 1.17) are: hardness 63HR15N, 0.2 - yield strength 525 MPa and total elongation 2.2% strain. Material which was exposed to a lower thermal fluence of 5.6 * 10 n/m , but with a thermal to fast neutron ratio of about 4, shows more radiation hardening : 67HR15N, 0.2 - yield strength 580 MPa and 1.5% total elongation. -5 -3 Fatigue crack growth rates range from 5 * 10 mm/cycle to 10 mm/cycle for AK ranging from 8 to 20 MPa)|m. The most highly exposed (7.5 * 10 n/m ) material shows accelerated fatigue crack growth due to unstable crack extension at AK of about 15 Mpa^m. The lowermost meaningful measure of plane strain fracture toughness is 18 MPa^m. Except for the fracture toughness which is a factor of about 3 higher the results show reasonable agreement with the expected mechanical properties estimated in the "safe end-of-life" assessment of the old HFR vessel. -

Planning for Seafood Freezing
TTTTTTTTTTT Planning for Seafood Freezing Edward KOLBE Donald KRAMER MAB-60 2007 Alaska Sea Grant College Program University of Alaska Fairbanks Fairbanks, Alaska 99775-5040 (888) 789-0090 Fax (907) 474-6285 www.alaskaseagrant.org TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT Elmer E. Rasmuson Library Cataloging-in-Publication Data: Kolbe, Edward. Planning for seafood freezing ⁄ Edward Kolbe and Donald Kramer. – Fairbanks, Alaska : Alaska Sea Grant College Program, University of Alaska Fairbanks, 2007 126 p. : 51 ill. ; cm. (Alaska Sea Grant College Program, University of Alaska Fairbanks ; MAB-60) Includes bibliographical references and index. 1. Frozen seafood—Preservation—Handbooks, manuals, etc. 2. Seafood— Preservation—Handbooks, manuals, etc. 3. Cold storage—Planning—Handbooks, manuals, etc. 4. Fishery management—Handbooks, manuals, etc. 5. Refrigeration and refrigeration machinery—Handbooks, manuals, etc. 6. Frozen fishery products—Handbooks, manuals, etc. I. Title. II. Kramer, Donald E. III. Series: Alaska Sea Grant College Program ; MAB-60. SH336.F7 K65 2007 ISBN 1-56612-119-1 Credits The work for this book was funded in part by the NOAA Office of Sea Grant, U.S. Department of Commerce, under grants NA76RG0476 (OSU), NA86RG0050 (UAF), and NA76RG0119 (UW); projects A/ESG-3 (OSU), A/151-01 (UAF), and A/FP-7 (UW), and by appropriations made by the Oregon, Alaska, and Washington state legislatures. Publishing is supported by grant NA06OAR4170013, project A/161-01. Sea Grant is a unique partnership with public and private sectors, combining research, education, and technology transfer for public service. This national network of universities meets the changing environmental and economic needs of people in our coastal, ocean, and Great Lakes regions. -

Heat Treating of Aluminum Alloys
ASM Handbook, Volume 4: Heat Treating Copyright © 1991 ASM International® ASM Handbook Committee, p 841-879 All rights reserved. DOI: 10.1361/asmhba0001205 www.asminternational.org Heat Treating of Aluminum Alloys HEAT TREATING in its broadest sense, • Aluminum-copper-magnesium systems The mechanism of strengthening from refers to any of the heating and cooling (magnesium intensifies precipitation) precipitation involves the formation of co- operations that are performed for the pur- • Aluminum-magnesium-silicon systems herent clusters of solute atoms (that is, the pose of changing the mechanical properties, with strengthening from Mg2Si solute atoms have collected into a cluster the metallurgical structure, or the residual • Aluminum-zinc-magnesium systems with but still have the same crystal structure as stress state of a metal product. When the strengthening from MgZn2 the solvent phase). This causes a great deal term is applied to aluminum alloys, howev- • Aluminum-zinc-magnesium-copper sys- of strain because of mismatch in size be- er, its use frequently is restricted to the tems tween the solvent and solute atoms. Conse- specific operations' employed to increase quently, the presence of the precipitate par- strength and hardness of the precipitation- The general requirement for precipitation ticles, and even more importantly the strain hardenable wrought and cast alloys. These strengthening of supersaturated solid solu- fields in the matrix surrounding the coher- usually are referred to as the "heat-treat- tions involves the formation of finely dis- ent particles, provide higher strength by able" alloys to distinguish them from those persed precipitates during aging heat treat- obstructing and retarding the movement of alloys in which no significant strengthening ments (which may include either natural aging dislocations. -

Ferrous Friction Stir Weld Physical Simulation A
FERROUS FRICTION STIR WELD PHYSICAL SIMULATION A Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Seth Jason Norton, M.S. ******** The Ohio State University 2006 Dissertation Committee: Approved by Dr. John Lippold, Adviser Dr. David Dickinson Adviser Dr. Charles Albright Welding Engineering Graduate Program ii ABSTRACT Traditional fusion welding processes have several drawbacks associated with the melting and solidification of metal. Weld defects associated with the solidification of molten metal may act as initiation sites for cracks. Segregation of alloying elements during solidification may cause local changes in resistance to corrosion. The high amount of heat required to produce the molten metal in the weld can produce distortion from the intended position on cooling. The heat from the electric arc commonly used to melt metal in fusion welds may also produce metal fumes which are a potential health hazard. Friction stir welding is one application which has the potential to make full thickness welds in a single pass, while eliminating fume, reducing distortion, and eliminating solidification defects. Currently the friction stir welding process is used in the aerospace industry on aluminum alloys. Interest in the process by industries which rely on iron and its alloys for structural material is increasing. While friction stir welding has been shown to be feasible with iron alloys, the understanding of friction stir welding process effects on these materials is in its infancy. This project was aimed to better that understanding by developing a procedure for physical simulation of friction stir welding. -

Aluminum and Aluminum-Alloy Extruded Bars, Rods, Wire, Profiles
Designation: B 221 – 05a Standard Specification for Aluminum and Aluminum-Alloy Extruded Bars, Rods, Wire, Profiles, and Tubes1 This standard is issued under the fixed designation B 221; the number immediately following the designation indicates the year of original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript epsilon (e) indicates an editorial change since the last revision or reapproval. This standard has been approved for use by agencies of the Department of Defense. 1. Scope* B211 Specification for Aluminum and Aluminum-Alloy 1.1 This specification2 covers aluminum and aluminum- Bar, Rod, and Wire alloy extruded bar, rod, wire, profile, and tube in the aluminum B 241/B 241M Specification for Aluminum and Aluminum- alloys (Note 1) and tempers shown in Table 2. Alloy Seamless Pipe and Seamless Extruded Tube B 429 Specification for Aluminum-Alloy Extruded Struc- NOTE 1—Throughout this specification, the use of the term alloy in the tural Pipe and Tube general sense includes aluminum as well as aluminum alloy. B 557 Test Methods of Tension Testing Wrought and Cast NOTE 2—For rolled or cold-finished bar and rod refer to Specification B211, for drawn tube, Specification B 210, for structural pipe and tube, Aluminum- and Magnesium-Alloy Products Specification B 429, and for seamless pipe and tube, Specification B 594 Practice for Ultrasonic Inspection of Aluminum- B 241/B 241M. Alloy Wrought Products for Aerospace Applications 1.2 Alloy and temper designations are in accordance with B 660 Practices for Packaging/Packing of Aluminum and ANSI H35.1. -

Establishing a Correlation Between Interfacial Microstructures and Corro- Sion Initiation Sites in Al/Cu Joints by SEM-EDS and AFM-SKPFM
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Sarvghad Moghaddam, Madjid, Parvizi, R., Davoodi, Ali, Haddad- Sabzevar, Mohsen, & Imani, Amin (2014) Establishing a correlation between interfacial microstructures and corro- sion initiation sites in Al/Cu joints by SEM-EDS and AFM-SKPFM. Corrosion Science, 79, pp. 148-158. This file was downloaded from: https://eprints.qut.edu.au/95930/ c Consult author(s) regarding copyright matters This work is covered by copyright. Unless the document is being made available under a Creative Commons Licence, you must assume that re-use is limited to personal use and that permission from the copyright owner must be obtained for all other uses. If the docu- ment is available under a Creative Commons License (or other specified license) then refer to the Licence for details of permitted re-use. It is a condition of access that users recog- nise and abide by the legal requirements associated with these rights. If you believe that this work infringes copyright please provide details by email to [email protected] License: Creative Commons: Attribution-Noncommercial-No Derivative Works 2.5 Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1016/j.corsci.2013.10.039 Accepted Manuscript Establishing a Correlation between Interfacial Microstructures and Corrosion Initiation Sites in Al/Cu Joints by SEM-EDS and AFM-SKPFM M. -

Specifications of 2 Kelvin J-T Heat Exchanger
Specification of Brazed aluminium plate fin heat exchanger A. Scope of Supply (Qty-1 No.) Fabrication and supply of brazed aluminium plate fin heat exchanger as per parameters described in Table-1. B. Bidder Qualification Criteria 1. Original Manufacturer must have enough experience and knowledge of complete manufacturing cycle of brazed plate fin heat exchanger. He must have experience in supplying brazed plate fin heat exchanger. The supplier shall submit/upload supporting documents (scan copy of purchase order) for the same along with bid. 2. If the bidder is not the original manufacturer then he must submit the details of original manufacturer/fabricator. And quotation will be accepted only when the original manufacturer/fabricator qualify the above mentioned criteria. C. Design Details and Requirements 1. The manufacturer will prepare all the fabrication drawings according to the details mentioned in Table 1, with necessary flow distributor,headers and nozzles for passage of low pressure and high pressure stream. It is manufacturer’s responsibility to prepare final fabrication drawings of complete unit with all necessary details and get written approval from purchaser before commencing the fabrication. 2. The heat exchanger shall be made as per the standard tolerances given in ALPEMA code for the external dimensions of brazed Aluminium plate-fin heat exchangers. Table 1. Details of various parameters of plate fin heat exchanger Parameters Details Working Fluid (hot and cold side) Helium Heat Exchanger core Material ASTM 3003 Aluminium alloy Headers and Nozzle Material ASTM 5083/5454 Aluminium alloy Mass flow rate 6 g/s Operating pressure 6 bara maximum for hot side 16 mbara for cold side Fin type Offset Serrated Fin Fin density 14 fins/inch (551 fins/m) +0.2mm Fin height for hot side 3.8mm−0mm Fin height for cold side +0.2mm 6.5mm−0mm Fin Flow length Minimum 3mm and Maximum 5 mm Fin Thickness 0.2 mm Parting sheet thickness 0.8 mm Side bar thickness 8 mm No. -

A Survey of Al7075 Aluminium Metal Matrix Composites
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2013): 4.438 A Survey of Al7075 Aluminium Metal Matrix Composites Rajendra .S .K1, Ramesha .C .M2 1Research Scholar, Jain University, Bengaluru, Department of Industrial Engineering and Management, Dr. Ambedkar Institute of Technology, Bengaluru 2Department of Mechanical Engineering, M S Ramaiah Institute of Technology, Bengaluru Abstract:A composite material is a combination of two or more chemically distinct and insoluble phases; its properties and structural performance are superior to those of the constituents acting independently. Metals and ceramics, as well, can be embedded with particles or fibers, to improve their properties; these combinations are known as Metal-Matrix composites. Aluminum 7075 alloy constitutes a very important engineering material widely employed in the aircraft and aerospace industry for the manufacturing of different parts and components. It is due to its high strength to density ratio that it a sought after metal matrix composite. In this paper we present a survey of Al 7075 Metal Matrix Composites. Keywords: Metal Matrix Composites (MMC’s), Aluminium Metal Matrix, Beryl, Al7075, Aluminium alloy 1. Introduction Aluminium alloy 7075 is an aluminium alloy, with zinc as the primary alloying element. It is strong, with a strength The effects of research in Aluminium based Metal Matrix comparable to many steels, and has good fatigue strength and Composites (MMC’s) are far reaching these days. These average machinability, but has less resistance to corrosion composites find various applications in the automobile than many other Al alloys. Its relatively high cost limits its industry, the aerospace industry and in defence and marine use to applications where cheaper alloys are not suitable. -
![191002 Knowledge: K1.02 [2.7/2.9] Qid: P6 (B1806)](https://docslib.b-cdn.net/cover/3766/191002-knowledge-k1-02-2-7-2-9-qid-p6-b1806-923766.webp)
191002 Knowledge: K1.02 [2.7/2.9] Qid: P6 (B1806)
NRC Generic Fundamentals Examination Question Bank--PWR May 2020 TOPIC: 191002 KNOWLEDGE: K1.02 [2.7/2.9] QID: P6 (B1806) Density input is normally used in steam flow instruments to convert __________ into __________. A. mass flow rate; volumetric flow rate B. volumetric flow rate; mass flow rate C. mass flow rate; differential pressure D. differential pressure; volumetric flow rate ANSWER: B. TOPIC: 191002 KNOWLEDGE: K1.02 [2.7/2.9] QID: P305 (B2906) If the steam pressure input to a density-compensated steam flow instrument fails high, the associated flow rate indication will... A. decrease, because the density input has decreased. B. increase, because the density input has decreased. C. decrease, because the density input has increased. D. increase, because the density input has increased. ANSWER: D. -1- Sensors and Detectors NRC Generic Fundamentals Examination Question Bank--PWR May 2020 TOPIC: 191002 KNOWLEDGE: K1.02 [2.7/2.9] QID: P406 (B1606) The density compensating input to a steam flow instrument is used to convert volumetric flow rate into… A. velocity flow rate. B. gallons per minute. C. mass flow rate. D. differential flow rate. ANSWER: C. -2- Sensors and Detectors NRC Generic Fundamentals Examination Question Bank--PWR May 2020 TOPIC: 191002 KNOWLEDGE: K1.02 [2.7/2.9] QID: P1212 If the steam pressure input to a density-compensated steam flow instrument fails low, the indicated flow rate will... A. increase, because the density input has increased. B. decrease, because the density input has increased. C. increase, because the density input has decreased. D. decrease, because the density input has decreased. -

Machining of Aluminum and Aluminum Alloys / 763
ASM Handbook, Volume 16: Machining Copyright © 1989 ASM International® ASM Handbook Committee, p 761-804 All rights reserved. DOI: 10.1361/asmhba0002184 www.asminternational.org MachJning of Aluminum and AlumJnum Alloys ALUMINUM ALLOYS can be ma- -r.. _ . lul Tools with small rake angles can normally chined rapidly and economically. Because be used with little danger of burring the part ," ,' ,,'7.,','_ ' , '~: £,~ " ~ ! f / "' " of their complex metallurgical structure, or of developing buildup on the cutting their machining characteristics are superior ,, A edges of tools. Alloys having silicon as the to those of pure aluminum. major alloying element require tools with The microconstituents present in alumi- larger rake angles, and they are more eco- num alloys have important effects on ma- nomically machined at lower speeds and chining characteristics. Nonabrasive con- feeds. stituents have a beneficial effect, and ,o IIR Wrought Alloys. Most wrought alumi- insoluble abrasive constituents exert a det- num alloys have excellent machining char- rimental effect on tool life and surface qual- acteristics; several are well suited to multi- ity. Constituents that are insoluble but soft B pie-operation machining. A thorough and nonabrasive are beneficial because they e,,{' , understanding of tool designs and machin- assist in chip breakage; such constituents s,~ ,.t ing practices is essential for full utilization are purposely added in formulating high- of the free-machining qualities of aluminum strength free-cutting alloys for processing in alloys. high-speed automatic bar and chucking ma- Strain-hardenable alloys (including chines. " ~ ~p /"~ commercially pure aluminum) contain no In general, the softer ailoys~and, to a alloying elements that would render them lesser extent, some of the harder al- c • o c hardenable by solution heat treatment and ,p loys--are likely to form a built-up edge on precipitation, but they can be strengthened the cutting lip of the tool.