Aluminum and Aluminum Alloys
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Connor Final Highlighted
Preliminary Development of a PPAM Actuated Pediatric Prosthetic Ankle Connor McNamara-Spackman A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science (Bioengineering) at the University of Otago, Dunedin, New Zealand. February 2021 Abstract The purpose of this research was to develop a preliminary design of a powered pediatric prosthetic ankle. Previous research identified the health risk of improper gait cycle and the lack of powered prosthetic ankle options for children. Costs for powered prosthetic ankles are too high (upwards of $5000 NZD), the sizes are too large and the weight is too significant for a child to benefit from. Current technologies for ankle joint actuation and materials for the prosthetic structure were evaluated and a conclusion of utilizing PPAMs was chosen due to their ability to generate the required 300 N of contraction force. CAD was used to model the structure of a prosthetic ankle and evaluate the FOS of the different material combinations while under static loading and fatigue simulations. HDPE and UHMWPE failed to withstand the simulations, while the aluminium alloy and stainless steel showed minimal faults from the simulations. MatLab was used to simulate the desired PPAM dimensions of 100 mm to determine the contraction force and contraction percentage that can be generated by the PPAM. The smallest PPAM found in research was 110 mm and showed promising results from their mathematical modeling. The overall height of the prosthetic was no greater than 110 mm and the membrane length of the PPAM was no greater than 100 mm, while successfully producing more than 300 N during contraction. -

Reduction of Out-Of-Plane Distortion in Fillet Welded High Strength Aluminum
Calhoun: The NPS Institutional Archive Theses and Dissertations Thesis Collection 1974 Reduction of out-of-plane distortion in fillet welded high strength aluminum. Henry, Robert W. Massachusetts Institute of Technology http://hdl.handle.net/10945/17204 REDUCTION OF OUT-OF-PLANE DISTORTION IN FILLET WELDED HIGH STRENGTH ALUMINUM Robert W. Henry il Postgraduate School vionterey, California 93940 REDUCTION OF OUT-OF-PLANE DISTORTION IN FILLET WELDED HIGH STRENGTH ALUMINUM BY Robert W. Henry B.S., U.S. Coast Guard Academy (1969) SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREES OF MASTER OF SCIENCE IN OCEAN ENGINEERING AND MASTERS OF SCIENCE IN MECHANICAL ENGINEERING at the Massachusetts Institute of Technology May, 1974 Tii-c DUDLEY KNOX LIBRARY ^STGRADL'ATE SCHOOU - 93940 REDUCTION OF OUT-OF-PLANE DISTORTION IN FILLET WELDED HIGH STRENGTH ALUMINUM by Robert W. Henry Submitted to the Department of Ocean Engineering in May, 1974, in partial fulfillment of the requirements for the degree of Master of Science in Ocean Engineering and Masters of Science in Mechanical Engineering. ABSTRACT Out-of-plane distortion caused by angular changes at fillet welds in aluminum structural panels was examined from two viewpoints. In the first phase of this work a series of experiments was conducted to examine elastic-plastic prestraining of test specimens to be fillet welded as a means of reducing out-of -plane distortion. Data gathered from these tests was correlated with previous experiments in the use of aluminum. A guide in the use of elastic-plastic prestrain- ing for 3/8" and 1/2" was developed. In phase two of this work a two-dimensional program was adapted to the structural aluminum panels used in phase one and tested for accuracy. -

Alcotec Aluminum Technical Guide
AlcoTec Aluminum Technical Guide Contents AlcoTec Aluminum Wire & Equipment Technical Guide Table of Contents AlcoTec Aluminum Wire & Equipment Technical Guide ......................................................................................................... 1 Table of Contents ................................................................................................................................................................ 1 Environmental Health and Safety ......................................................................................................................................... 3 Technical Services Heat Treatable & Non-Heat Treatable Base & Fillers ............................................................................................................. 6 Filler Alloys: Chemical Composition Limits & Physical Properties ......................................................................................... 7 Conversion Factors ............................................................................................................................................................ 7 Welded Joint Strength ......................................................................................................................................................... 8 Typical Tensile Properties - Groove Welds ............................................................................................................................ 9 Weld Profiles ..................................................................................................................................................................... -

Parshwamani Metals
+91-8048554624 Parshwamani Metals https://www.indiamart.com/parshwamanimetals/ Parshwamani Metals is one of the leading manufacturers, supplier and traders of Industrial Metal Tube, Beryllium Product, Shim Sheet, SS Round And Square Bar, Aluminium Products, Aluminum Bronze Products etc. About Us Parshwamani Metals was established in the year 2015 as a professionally managed Manufacturer, Trader and Wholesaler specialized in providing premium grade Copper and Brass Metals Products. Today, we endeavor to revolutionize the industry by fabricating a wide gamut of quality products, which includes Brass Products, Copper Products and Copper Alloy. Our claim to success is hallmarked by the offered quality products that gained us huge recognizance for its high strength, wear and tear resistance, accurate dimensions, flexibility and durable finish. Our products find their wide applications in architectural fittings, hardware and telecommunication. Owing to swift delivery schedules, easy payment modes and overt business practices, we have been successful in earning huge client base. We deal in Jindal Brand. Our efforts are determined with the objective of industrial leadership that equips our team members to manufacture customized products. And, to achieve this, we have developed modernized R&D centers and cutting edge manufacturing facilities. Furthermore, the facility is divided into various functional units like procurement, engineering, production, research & development, quality-testing, warehousing & packaging etc. Our organization is backed -

Downloaded From
Proceedings of The Intl. Conf. on Information, Engineering, Management and Security 2014 [ICIEMS 2014] 144 Effects of Combined Addition of Aluminum Oxide, Fly Ash, Carbon and Yttrium on Density and Hardness of ZA27 Zinc Alloy A. K. Birru a* , R. Manohar Reddy a, B. Srinivas a, K. Balachandan Reddy a and K. Nithin a aDepartment of Mechanical Engineering, Christu Jyothi Institute Of Technology & Science, Jangaon, Warangal, India Abstract: ZA-27 alloy plays a vital role in ZA family of alloys with a high strength and pinnacle applications in manufacturing. The research papers emphasized to enhance hardness and minimize the density of the aforesaid alloy with combined addition of Al 203, fly ash, carbon and yttrium as reinforcements. Hence we observed that with density was gradually decreased at 7% with 5% Al 2O3, 0.15% carbon and 0.01% Yttrium addition. Similarly, further decreased density at 10% with 7.5% Al 2O3, 0.25% carbon and 0.05% Yttrium. However, hardness was initial increased more than 11% with 5% Al 2O3, 0.15% carbon and 0.01% Yttrium. Conversely, hardness was slightly decreased at 5% with 7.5% Al 2O3, 0.25% carbon and 0.05% Yttrium. Keywords: Aluminum oxide; Flyash; Carbon; Yttrium; Density; Hardness. 1. Introduction Zinc alloys with higher aluminium content (25-27 wt. %) obtained by conventional processes of melting and casting, are applied in various fields, particularly in automobile industry, because of their good mechanical, technological and economical properties. Lim Ying Pio et al. [1] conducted the experimentation of LM6 Al- Si alloy on a sand casting of different modulus, the addition level of Al5Ti1B into the melt ranges from 0 wt. -
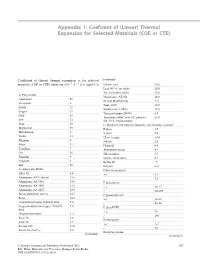
Linear) Thermal Expansion for Selected Materials (COE Or CTE
Appendix 1: Coefcient of (Linear) Thermal Expansion for Selected Materials (COE or CTE) Coefficient of (linear) thermal expansion, α, for selected (continued) − − materials (COE or CTE) (units are ×10 6 °C 1 (i.e. ppm/°C)) Indium–lead 33.0 Lead (95 %) tin solder 28.0 Tin–lead solder 60/40 25.0 A. Pure metals Magnesium, AZ31B 26.0 Aluminium 25 Ni-clad Molybdenum 5–6 Chromium 6 Steel, 1020 12.0 Cobalt 12 Stainless steel (18-8) 17.0 Copper 17 Tungsten/copper (90/10) 6.5 Gold 14 Aluminium MMC with SiC particles 6–14 Iron 12 (80–50 % reinforcement) Lead 29 C. Insulators and substrate materials (for electronic systems)a Magnesium 25 E glass 5.5 Molybdenum 5 S glass 2.6 Nickel 13 Glass–ceramic >3.0 Platinum 9 Silicon 2.6 Silver 19 Diamond 0.9 Tantalum 7 Aluminium nitride 4.5 Tin 20 Silicon nitride 3.7 Titanium 9 Quartz, fused silica 0.5 Tungsten 5 Kevlar 49 –5 Zinc 35 Beryllia 6–9 B. Alloys and MMCs Cubic boron nitride Alloy 42 4.4 x–y 3.7 Aluminium (40 % silicon) 13.5 z 7.2 Aluminium, AA 6061 23.6 E glass/epoxy Aluminium, AA 3003 23.2 x–y 14–17 Aluminium, AA 2017 22.9 z 80–280 Boron aluminium (20 %) 12.7 E glass/polyimide Brass 18.0 x–y 12–16 Copper/invar/copper 20/60/20 thick 5.8 z 40–80 Copper/molybdenum/copper 20/60/20 7.0 E glass/PTFE thick x–y 24 Graphite/aluminium 4–6 z 260 Invar 36 1.6 Kevlar/epoxy Invar 42 4.5 x–y 5–7 Inconel 600 13.0 z 70 Kovar (Fe–Ni–Co) 5.0 (continued) Kevlar/polyimide (continued) © Springer International Publishing Switzerland 2016 557 B.D. -

Characteristics of Al-Si Alloys with High Melting Point Elements for High Pressure Die Casting
materials Article Characteristics of Al-Si Alloys with High Melting Point Elements for High Pressure Die Casting Tomasz Szymczak 1,* , Grzegorz Gumienny 1,* , Leszek Klimek 2 , Marcin Goły 3 , Jan Szymszal 4 and Tadeusz Pacyniak 1 1 Department of Materials Engineering and Production Systems, Lodz University of Technology, 90-924 Lodz, Poland; [email protected] 2 Institute of Materials Science and Engineering, Lodz University of Technology, 90-924 Lodz, Poland; [email protected] 3 Department of Physical & Powder Metallurgy, AGH University of Science and Technology, 30-059 Krakow, Poland; [email protected] 4 Department of Technical Sciences and Management, University of Occupational Safety Management in Katowice, 40-007 Katowice, Poland; [email protected] * Correspondence: [email protected] (T.S.); [email protected] (G.G.); Tel.: +48-426312276 (T.S.); +48-426312264 (G.G.) Received: 9 October 2020; Accepted: 29 October 2020; Published: 29 October 2020 Abstract: This paper is devoted to the possibility of increasing the mechanical properties (tensile strength, yield strength, elongation and hardness) of high pressure die casting (HPDC) hypoeutectic Al-Si alloys by high melting point elements: chromium, molybdenum, vanadium and tungsten. EN AC-46000 alloy was used as a base alloy. The paper presents the effect of Cr, Mo, V and W on the crystallization process and the microstructure of HPDC aluminum alloy as well as an alloy from the shell mold. Thermal and derivative analysis was used to study the crystallization process. The possibility of increasing the mechanical properties of HPDC hypoeutectic alloy by addition of high-melting point elements has been demonstrated. -

Tribological Behaviours Influenced by Surface Coatings and Morphology
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 7-11-2015 Tribological Behaviours Influenced yb Surface Coatings and Morphology Guang Wang University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Wang, Guang, "Tribological Behaviours Influenced yb Surface Coatings and Morphology" (2015). Electronic Theses and Dissertations. 5305. https://scholar.uwindsor.ca/etd/5305 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. Tribological Behaviours Influenced by Surface Coatings and Morphology By Guang Wang A thesis Submitted to the Faculty of Graduate Studies through the Department of Mechanical, Automotive & Materials Engineering in Partial Fulfillment of the Requirements for the Degree of Master of Applied Science at the University of Windsor Windsor, Ontario, Canada 2015 ©2015 Guang Wang Tribological Behaviours Influenced by Surface Coatings and Morphology By Guang Wang APPROVED BY: Dr. -

Aluminium Products Coil
Helping manufacturers across the globe achieve sustainable leaner manufacturing processes Aluminium Coil, Foil, Products Sheet & Wire Commercially Pure Aluminium Alloys Series 1000 Series 2000 Series 3000 Fast Series 4000 Series 5000 Turnaround Series 6000 Processing Series 7000 Series 8000 Clad Aluminium WIDE STOCK RANGE Low Width Thickness Ratio 3:1 unique to the industry (normal minimum is 8:1) Over 75 years Experience Knight Group Visit our websites: Main: www.knight-group.co.uk Offcuts: www.ksmdirect.co.uk www.pmdirect.be Head Office Linkside, Summit Road Cranborne Industrial Estate Potters Bar, Hertfordshire EN6 3JL United Kingdom Main Office : +44(0)1707 650251 Fax: +44(0)1707 651238 [email protected] Knight Strip Metals Ltd Sales, Processing & Warehouse Saltley Business Park Cumbria Way, Saltley Birmingham B8 1BH United Kingdom Telephone: +44 (0)121 322 8400 Fax: +44 (0)121 322 8401 Sales 08456 447 977 [email protected] Precision Metals EU Industriezone Mechelen-Noord (D) Omega Business Park Wayenborgstraat 25 2800 Mechelen Belgium Telephone: +32 (0) 15 44 89 89 Fax: +32 (0) 15 44 89 90 [email protected] The information contained herein is given in good faith and is based on our present knowledge and experience. However, no liability will be accepted by the Knight Group and its subsidiaries in respect of any action taken by any third party in reliance thereon. Any advice given by the Company to any third party is given for that party’s assistance only and without any liability on the part of the Company. The contents of this brochure are subject to change and the most recent edition of all Knight Group documentation can be found on our website or by written request. -

Planning for Seafood Freezing
TTTTTTTTTTT Planning for Seafood Freezing Edward KOLBE Donald KRAMER MAB-60 2007 Alaska Sea Grant College Program University of Alaska Fairbanks Fairbanks, Alaska 99775-5040 (888) 789-0090 Fax (907) 474-6285 www.alaskaseagrant.org TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT Elmer E. Rasmuson Library Cataloging-in-Publication Data: Kolbe, Edward. Planning for seafood freezing ⁄ Edward Kolbe and Donald Kramer. – Fairbanks, Alaska : Alaska Sea Grant College Program, University of Alaska Fairbanks, 2007 126 p. : 51 ill. ; cm. (Alaska Sea Grant College Program, University of Alaska Fairbanks ; MAB-60) Includes bibliographical references and index. 1. Frozen seafood—Preservation—Handbooks, manuals, etc. 2. Seafood— Preservation—Handbooks, manuals, etc. 3. Cold storage—Planning—Handbooks, manuals, etc. 4. Fishery management—Handbooks, manuals, etc. 5. Refrigeration and refrigeration machinery—Handbooks, manuals, etc. 6. Frozen fishery products—Handbooks, manuals, etc. I. Title. II. Kramer, Donald E. III. Series: Alaska Sea Grant College Program ; MAB-60. SH336.F7 K65 2007 ISBN 1-56612-119-1 Credits The work for this book was funded in part by the NOAA Office of Sea Grant, U.S. Department of Commerce, under grants NA76RG0476 (OSU), NA86RG0050 (UAF), and NA76RG0119 (UW); projects A/ESG-3 (OSU), A/151-01 (UAF), and A/FP-7 (UW), and by appropriations made by the Oregon, Alaska, and Washington state legislatures. Publishing is supported by grant NA06OAR4170013, project A/161-01. Sea Grant is a unique partnership with public and private sectors, combining research, education, and technology transfer for public service. This national network of universities meets the changing environmental and economic needs of people in our coastal, ocean, and Great Lakes regions. -

Review on Multi-Pass Friction Stir Processing of Aluminium Alloys
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 22 July 2020 doi:10.20944/preprints202007.0514.v1 Review Review on Multi-Pass Friction Stir Processing of Aluminium Alloys Oritonda Muribwathoho1, Sipokazi Mabuwa1* and Velaphi Msomi1 1 Cape Peninsula University of Technology, Mechanical Engineering Department, Bellville, 7535, South Africa; [email protected]; [email protected] * Correspondence: [email protected]; Tel.: 27 21 953 8778 Abstract: Aluminium alloys have evolved as suitable materials for automotive and aircraft industries due to their reduced weight, excellent fatigue properties, high-strength to weight ratio, high workability/formability, and corrosion resistance. Recently, the joining of similar and dissimilar metals have achieved huge success in various sectors. The processing of soft metals like aluminium, copper, iron and nickel have been fabricated using friction stir processing. Friction stir processing (FSP) is a microstructural modifying technique that uses the same principles as the friction stir welding technique. In the majority of studies on FSP, the effect of process parameters on the microstructure was characterized after a single pass. However, multiple passes of FSP is another method to further modify the microstructure in aluminium castings. This study is aimed at reviewing the impact of multi-pass friction stir processed joints of aluminium alloys and to identify a knowledge gap. From the literature that is available on multi-pass FSP, it has been observed that the majority of the literature focused on the processing of plates than the joints. There is limited literature reporting on multi-pass friction stir processed joints. This then creates a need to study further on multi-pass friction stir processing on dissimilar aluminium alloys. -

Mechanical Behaviors of Joining AL-Alloys Based FSW Parameters and Welding Tool Design Ahmed El-Keran1, Rania Mostafa2, Reham Al-Mahdy3*
97 International Journal of Scientific & Engineering Research, Volume 10, Issue 6, June-2019 ISSN 2229-5518 Mechanical behaviors of joining AL-Alloys based FSW parameters and welding tool design Ahmed El-Keran1, Rania Mostafa2, Reham Al-Mahdy3* Production Engineering and Mechanical Design Deptartment, Faculty of Engineering, Mansoura University, Mansoura, Egypt. 1Assoc. Professor, [email protected] 2Lecturer, [email protected] 3* (corresponding author), [email protected] Abstract— Friction Stir Welding is a solid-state welding process with specially rotating tool design. The heat is generated due to the friction between the workpiece-surface and the tool shoulder. The core benefit of FSW joint is to weld the material without achieving the fusion temperature so it’s allows to join almost types of aluminum alloys, even the ones categorized as non-weld able alloy by the traditional welding process such as fusion welding because of the hot cracking and unfortunate solidification microstructure in the welding zone. FSW is used in aerospace, automotive, marine industries, electronics etc. This paper introduces a literature review on FSW process and various researches in this field. The effect of the welding parameters such as tool rotation speed, welding speed, tool tilt angle and tool design are introduced. The article also reviews the FSW of similar and dissimilar aluminum alloys and the FSW benefits and applications. Key Words — Friction Stir Welding, Al-Alloys, Welding parameters, FSW Tools, solid-state welding process, Rotional speed, Welding speed, Mechanical behaviors. —————————— u —————————— 1 INTRODUCTION In 1991, Wayne Thomas at The Welding Institute TWI in- vented a new welding process that is used to joint two pieces 2 Process principles by soften the rejoin between them without melting.