Evaluation of Antioxidant Activity of Two Curcuma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
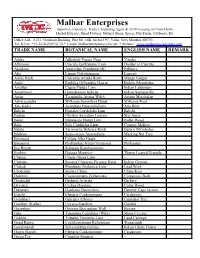
Trade Name Botanical Name English Name Remark
Malhar Enterprises Importers, Exporters, Traders, Indenting Agent & All Processing in Crude Herbs, Herbal Extracts, Dried Flowers, Natural Gums, Spices, Dry Fruits, Oil Seeds, Etc Office Add.: 2/233, Grohitam Building, Plot No. 14B, Sector 19C, Vashi, Navi Mumbai 400705. Tel. & Fax: +91-22-41236974/ 76 * E-mail: [email protected] * Website : www.malharent.tradeindia.com TRADE NAME BOTANICAL NAME ENGLISH NAME REMARK Adusa Adhatoda Vasica Nees Vasaka Aftimoon Cuscuta Epithymum Linn Dodder or Cuscuta Akarkara Anacyclus Pyrethrum DC Pellitory Alsi Linum Usitatissimum Linseed Amba Haldi Curcuma Amada Roxb Mango Ginger Amla Emblica Officinalis Gaertn Emblic Myrobalan Amaltas Cassia Fistula Linn Indian Laburnum Anantmool Hemidesmus Indicus Indian Sarsaparilla Arjun Terminalia Arjuna W&A Arjuna Myrobalan Ashwagandha Withania Somnifera Dunal Withania Root Atis Kadvi Aconitum Heterophyllum Atis Root Babchi Psoralea Corylifolia Linn Babchi Badian Illicium Anisatum Loureio Star Anise Bakul Mimusops Elengi Linn Bullet Wood Bala Sida Cordifolia Linn Country Mallow Behda Terminalia Belerica Roxb Beleric Myrobalan Bhilawa Semecarpus Anacardium Marking Nut Tree Bhringraj Eclipta Alba Hassk -- Bhuiamla Phyllanthus Niruri/ Fraternus Phyllantus Big Ringni Solanum Kanthocarpum -- Brahmi Bacopa Monnieri Thyme Leaved Gratiola Chaksu Cassia Absus Linn -- Chiraita Swertia Chirayita Fleming Karst Indian Gentian Chitrak Plumbago Zeylanica Linn. Lead Wort Chobchini Smilax China China Root Dalchini Cinnamomum Zeylanicum Cinnamom Bark Daruhaldi Berberis Aristata -

Genus Curcuma
JOURNAL OF CRITICAL REVIEWS ISSN- 2394-5125 VOL 7, ISSUE 16, 2020 A REVIEW ON GOLDEN SPECIES OF ZINGIBERACEAE FAMILY: GENUS CURCUMA Abdul Mubasher Furmuly1, Najiba Azemi 2 1Department of Analytical Chemistry, Faculty of Chemistry, Kabul University, Jamal Mina, 1001 Kabul, Kabul, Afghanistan 2Department of Chemistry, Faculty of Education, Balkh University, 1701 Balkh, Mazar-i-Sharif, Afghanistan Corresponding author: [email protected] First Author: [email protected] Received: 18 March 2020 Revised and Accepted: 20 June 2020 ABSTRACT: The genus Curcuma pertains to the Zingiberaceae family and consists of 70-80 species of perennial rhizomatous herbs. This genus originates in the Indo-Malayan region and it is broadly spread all over the world across tropical and subtropical areas. This study aims to provide more information about morphological features, biological activities, and phytochemicals of genus Curcuma for further advanced research. Because of its use in the medicinal and food industries, Curcuma is an extremely important economic genus. Curcuma species rhizomes are the source of a yellow dye and have traditionally been utilized as spices and food preservers, as a garnishing agent, and also utilized for the handling of various illnesses because of the chemical substances found in them. Furthermore, Because of the discovery of new bioactive substances with a broad range of bioactivities, including antioxidants, antivirals, antimicrobials and anti-inflammatory activities, interest in their medicinal properties has increased. Lack of information concerning morphological, phytochemicals, and biological activities is the biggest problem that the researcher encountered. This review recommended that collecting information concerning the Curcuma genus may be providing more opportunities for further advanced studies lead to avoid wasting time and use this information for further research on bioactive compounds which are beneficial in medicinal purposes KEYWORDS: genus Curcuma; morphology; phytochemicals; pharmacological 1. -

Flores Vivar S.Pdf
ENVIRONMENTAL CONDITIONS, CULTIVAR, AND PROPAGATION MATERIAL AFFECT RHIZOME YIELD AND POSTHARVEST QUALITY OF GINGER (ZINGIBER OFFICINALE ROSC.), GALANGAL (ALPINIA GALANGA LINN.), AND TURMERIC (CURCUMA SPP.) By SOFIA JESUS FLORES VIVAR A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2019 © 2019 Sofia J. Flores Vivar To my parents, siblings, and plant friends ACKNOWLEDGMENTS I would like to thank Dr. Rosanna Freyre and Dr. Paul Fisher for giving me the opportunity to work for their program initially as a Research Visiting Scholar. After that first experience, they let me continue working and guided me through my masters’ studies. I thank Dr. Sargent for his advice and help during my postharvest evaluations. Special thanks to present and past students from Dr. Fisher and Dr. Freyre’s labs for their help and support. Thanks to Victor Zayas, Erin Yafuso, Ulrich Adegbola, George Grant, Jonathan Clavijo, Henry Kironde, and Nicholas Genna for being amazing friends. I thank Brian Owens, Mark Kann and their teams for always being there to help me in the greenhouse and field. I would like to thank Dr. Pearson and his team from Mid-Florida Research and Education Center in Apopka for letting me work in their lab and providing instruction to perform the chemical analyses of my plants. I thank Dr. Rathinasabapathi for his advice and willingness to help me with the analysis of my rhizomes. I would like to thank Dr. Gomez for her friendship and unwavering support. Thanks to James Colee from UF Agriculture Statistics for support with statistical analysis. -

Phytochemical and Pharmacological Properties of Curcuma Aromatica Salisb (Wild Turmeric)
Journal of Applied Pharmaceutical Science Vol. 10(10), pp 180-194, October, 2020 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2020.1010018 ISSN 2231-3354 Phytochemical and pharmacological properties of Curcuma aromatica Salisb (wild turmeric) Nura Muhammad Umar1, Thaigarajan Parumasivam1*, Nafiu Aminu2, Seok-Ming Toh1 1School of Pharmaceutical Sciences, Discipline of Pharmaceutical Technology, Universiti Sains Malaysia (USM), 11800, Pulau Pinang, Malaysia. 2Department of Pharmaceutics and Pharmaceutical Microbiology, Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria. ARTICLE INFO ABSTRACT Received on: 11/06/2020 Curcuma aromatica Salisb. (C. aromatica) is commonly known as wild turmeric. Curcuma aromatica is an essential Accepted on: 04/08/2020 herbal plant and it has been extensively used in traditional medicine for centuries. It has been used for the treatment of Available online: 05/10/2020 gastrointestinal ailments, arthritic pain, inflammatory conditions, wounds, skin infections, and insect bites. This article aims to review the phytochemical and pharmacological aspects of C. aromatica and to provide a guide and insight for further studies. Electronic repositories, including Web of Science, Google Scholar, ProQuest, Science Direct, Key words: Scopus, and PubMed, were searched until December 2019 to identify studies relating to C. aromatica. A systematic Pharmacological activity, analysis of the literature on pharmacognostical, physicochemical, and nutritional contents, bioactive compounds, and phytochemical constituents, biological activities of C. aromatica was carried out, and ideas for future studies were also coined. A total of 157 Curcuma aromatica, articles concerning in vitro or in vivo (or both) researches on C. aromatica have been evaluated. Analyses of the data Zingiberaceae. -

Development and Evaluation of Carminative Herbal Chewable Tablets Based on Turmeric, Fennel Seed, and Mango Ginger Bapan Banik*, Ankita Sharma, Absana Nasrin
ORIGINAL ARTICLE e-ISSN: 2349-0659 p-ISSN; 2350-0964 Development and Evaluation of Carminative Herbal Chewable Tablets Based on Turmeric, Fennel Seed, and Mango Ginger Bapan Banik*, Ankita Sharma, Absana Nasrin ABSTRACT The present study aimed at the formulation of carminative herbal chewable tablets using herbal constituents. Curcuma longa (turmeric), Foeniculum vulgare (fennel), and Curcuma amada (mango ginger) are the most celebrated herbs in Indian system of traditional medicine. In the present research work, oral chewable tablets were prepared by direct compression and wet granulation method incorporating these three herbs. In both the methods, the powder of turmeric, fennel seed, and ginger mango was prepared initially and it was mixed with additives and preservatives. Physicochemical analysis of the individual drugs, pre-formulation studies, and post-formulation standardization was done to evaluate the quality and purity of the conformation. In the pre-formulation study, it was observed that all the parameters checked for the ingredients were within standard range. Thus, the ingredients were processed for preparing tablets following IP. During the evaluation of tablets, it was found that all the prepared batches of tablets were within the standard range of chewable tablet parameters. Thus, considering these values and following the IP, we found that the chewable tablet that was prepared without altering its therapeutic property was satisfactory with general characteristics of tablet, namely, hardness, disintegration time, friability, and weight variation. The formulation was tested for common people with respect to taste; odor and time required for complete chewing and showed that it can be accepted for the present trends of newer drug delivery dosage forms. -

(12) Patent Application Publication (10) Pub. No.: US 2016/0220627 A1 MELNICK Et Al
US 2016O220627A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0220627 A1 MELNICK et al. (43) Pub. Date: Aug. 4, 2016 (54) EXTRACTS OF CURCUMAAMADA AND (57) ABSTRACT USES THEREOF (71) Applicants: DHARMA BIOMEDICAL, LLC, The invention concerns carbon dioxide extracts of Curcuna Miami, FL (US); FLAVEX amada (mango ginger), including Supercritical carbon diox NATUREXTRAKTE GMBH, ide extracts of Canada; methods for their production; com Rehlingen (DE) positions comprising the extracts; methods for treating or (72) Inventors: STEVEN J. MELNICK, CORAL delavingR E. (th OSUt Oof conditiN 1OS i h in 11 pproliferati GABLES, FL (US); KARL-WERNER IN inst l 1. al Ion, ec In 'E, 1p1 QUIRIN, BECKINGEN (DE); emia, nypercno esterolem1a, ypertrig yceridemia, nyperg CHEPPAIL RAMACHANDRAN, lycemia, platelet hyper-aggregation, immune disorder Such MIAMI, FL (US); MELVIN as autoimmune disorder, or neurodegenerative condition; and ROTHBERG, WESTON, FL (US) methods for inhibiting expression of Bcl-2. Bak, and p53 9. enes; inhibitingg expressioneXp of the COX-2 and NF-kB genes,9. (21) Appl. No.: 15/011,628 inhibiting production of phosphorylated target of rapamycin 1-1. (TOR), modulating AMP-activated protein kinase (AMPK), (22) Filed: Jan. 31, 2016 inhibiting protein kinase B (AKT) signaling, modulating the Related U.S. Application Data RaS/PI3K/PTENEMSso mTOR signalingRNA. andpathway. tly Another R (60) Eyal application No. 62/110,062, filed on Jan. aspect of the invention concerns a method for inhibiting con s tamination, comprising applying the extract or composition Publication Classification of the invention to a surface. Another aspect of the invention concerns a method for ppromoting glongeV1ty longevity of a cell invitro or (51) Int. -

Curcuma Amada Roxb
NEWSLETTER Knowledge of herbs – Curcuma amada Roxb . Drug consists of the dried rhizome of Curcuma amada Roxb.; Fam. Zingiberaceae, the plant grows wild in parts of Bengal, Konkan and Tamil Nadu. It is also cultivated throughout India. Other name:- Beng.- Amada Eng.- Mango-ginger Hindi- Am haldi, Ama haldi Kan.- Ambahalad Mal. Mannayinci Mar. Amba haladi Tam. Mangai inji, Mankayyinci Tel. Mamidiallam Guj. Ambahalad Scientific classification:- Kingdom: Plantae Order: Poales Family: Cyperaceae Genus: Cyperus Species: C. rotundus Description:- A rhizomatous aromatic herb; buff coloured rhizome. Laterally flattened, longitudinally wrinkled, 2 to 6 cm long, 0.5 to 2 cm in diameter demarcated into nodal and inter nodal region, remnant of scaly leaves arranged circularly giving the appearance of growth rings; circular, punctate scars on the surface; roots long. Tapering, thread like, yellowish brow; fracture short often fibrous, raw mango-like odour and taste pungent. Medicinal Uses: Carminative, stomachic, appetizer, expectorant, antipyretic, anti-inflammatory. Specific in rheumatism and inflammation of liver; rheumatism; in contusions and sprains. Chemical constituents: Essential oil 1 % (1,8-cineole, myrcene), curcumene, pinene, camphor, α- and β- curcumene, turmerone and a phytosterol. 1,8 – cineole myrcene Adulterants/Substitutes: C.amada is adulterated with C. zedoaria (Berg.) Rose has oval club shaped pieces of rhizome. The external surface is yellowish brown with ring like root scars, while the cut surface is deep orange in colour. The odour is camphoraceous and the taste is pungent. Safety aspects: The drug is traditionally considered to be safe in the dosage mentioned. Reference:- 1) Dutta S, Tayal JN. Chemical examination of the essential oil derived from the rhizomes of Curcuma amada Roxb. -

Extracts, Seeds and Planting Material Are Available for All. CO2 Extracts
Extracts, Seeds and Planting material are available for all. CO2 extracts, Oils and Oleoresins are available for all herbs marked * Herbs Convolvulus Ocimum kilimandscharicum (Karpur Abrus precatorius (Rati Gunj) pluricaulis Tulsi) (Shankhpushpi) Abutilon indicum (Atibala) Corchorus depressus Ocimum sanctum (Tulsi)* Curculigo orchioides Acacia Arabica (Babul) Origanum majorana (Marjoram)* (Musli) Curcuma amada Acacia catechu (Khadir) Origanum vulgare (Oregano)* (Amra Haridra)* Curcuma aromatica Acacia concina (Shikakai) Oroxylum indicum (Shyonak) (Van Haridra)* Acacia nilotica (Babul) Curcuma officinalis Peganum harmala (Harmal) Cymbopogon citratus Achyranthes aspera (Apamarg) Petroselinum crispum (Parsley) (Lemongrass)* Cymbopogon Acorus calamus (Vacha) flexuosus Piper longum (Pippali) (Lemongrass)* Cymbopogon martinii Adhatoda vasica (Vasa) Plantago ovata (Psyllium) (Palmarosa)* Cymbopogon Aegle marmelos (Bilva) winteriaruns Pluchea lanceolata (Rasna) (Citronella)* Cynodon dactylon Aerva persica (Bhadra) Plumbago zeylanica (Chitrak) (Durva) Cyperus rotundus Albizia lebbek (Shirish) Pogostemon cabin (Patchouli)* (Musta)* Datura metel, D. Aloe barbadensis (Ghrit Kumari) Pongamia pinnata, P. glabra (Karanj) fastuosa (Dhoorta) Desmostachya Alpinia galanga (Kolinjan)* Premna integrifolia (Agnimanta) bipinnata (Kusha) Dioscorea bulbifera Alstonia scholaris (Saptparni) Psidium guajava (Guava) (Varahikanda) Eclipta alba Ammi majus (Bishop's Weed) Psoralea corylifolia (Bakuchi) (Bhringraja) Embelia ribes Amorphophallus campanularus Pueraria -

The Genus Curcuma and Inflammation
plants Review The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives Md. Moshiur Rahaman 1, Ahmed Rakib 2 , Saikat Mitra 3, Abu Montakim Tareq 4 , Talha Bin Emran 5 , A. F. M. Shahid-Ud-Daula 1,*, Mohammad Nurul Amin 6,7,* and Jesus Simal-Gandara 8,* 1 Department of Pharmacy, Noakhali Science and Technology University, Noakhali 3814, Bangladesh; [email protected] 2 Department of Pharmacy, Faculty of Biological Sciences, University of Chittagong, Chittagong 4331, Bangladesh; [email protected] 3 Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka 1100, Bangladesh; [email protected] 4 Department of Pharmacy, International Islamic University Chittagong, Chittagong 4318, Bangladesh; [email protected] 5 Department of Pharmacy, BGC Trust University Bangladesh, Chittagong 4381, Bangladesh; [email protected] 6 Department of Pharmacy, Atish Dipankar University of Science and Technology, Dhaka 1230, Bangladesh 7 Pratyasha Health Biomedical Research Center, Dhaka 1230, Bangladesh 8 Nutrition and Bromatology Group, Department of Analytical and Food Chemistry, Faculty of Food Science and Technology, University of Vigo–Ourense Campus, E32004 Ourense, Spain * Correspondence: [email protected] (A.F.M.S.-U.-D.); [email protected] (M.N.A.); [email protected] (J.S.-G.) Abstract: The Curcuma genus has been extensively used for therapeutic purposes in traditional or folk medicine worldwide, including for its anti-inflammatory activity. Curcuma spp.’s active constituents, such as alkaloids, flavonoids, and terpenoids, can act on various targets in the signaling Citation: Rahaman, M.M.; Rakib, A.; pathway, restrain pro-inflammatory enzymes, lower the production of inflammatory cytokines and Mitra, S.; Tareq, A.M.; Emran, T.B.; chemokines, and reduce oxidative stress, which subsequently suppresses inflammatory processes. -

Family Zingiberaceae Compounds As Functional Antimicrobials, Antioxidants, and Antiradicals
Food ©2007 Global Science Books Family Zingiberaceae Compounds as Functional Antimicrobials, Antioxidants, and Antiradicals Supayang P. Voravuthikunchai Natural Products Research Center and Department of Microbiology, Faculty of Science, Prince of Songkla University, Thailand Correspondence : [email protected] ABSTRACT Increasing numbers of reported cases of food-associated infections and health problems associated with synthetic additives have led to a growing interest by consumers in ingredients from natural sources. Some members of the family Zingiberaceae have been extensively used as a condiment for flavoring as well as traditional medicines. These include Alpinia galanga (galanga), Boesenbergia pandurata (krachai), Curcuma amada (mango ginger), Curcuma longa (turmeric), Curcuma zedoria (zedoary), Kampferia galanga (proh hom), Zin- giber officinale (ginger), and Zingiber zerumbet (zerumbet ginger). Their antimicrobial activities against important foodborne pathogens including Bacillus cereus, Campylobacter jejuni, Clostridium botulinum, Clostridium perfringens, Escherichia coli, Listeria monocyto- genes, Salmonella spp., Shigella spp., Staphylococcus aureus, Vibrio spp., Yersinia enterocolitica, Hepatitis A Norwalk virus, Entamoeba histolytica, and Giardia lamblia are outlined. In addition to the antimicrobial activities against a wide range of microorganisms, their antioxidant activities have been documented. The potential uses of these plant species as food preservatives are discussed. _____________________________________________________________________________________________________________ -

Biochemical Studies on Curcuma Amada Extracts
Available online a t www.scholarsresearchlibrary.com Scholars Research Library Archives of Applied Science Research, 2014, 6 (2): 18-23 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-508X CODEN (USA) AASRC9 Biochemical studies on Curcuma amada extracts P. Venugopalan *, Soumya Mohan and Deepthi T. V. Department of Chemistry, Sree Neelakanta Government Sanskrit College Pattambi ,Palakkad, Kerala, India _____________________________________________________________________________________________ ABSTRACT Naturally occurring antioxidants have considerable importance in medicine and in food processing. In this work, the antioxidant activity of dried rhizomes extract of the spice Curcuma amada (Mango ginger), a unique spice having morphological resemblance with ginger (Zingiber officinale) but imparts a raw mango (Mangifera indica) flavour is studied by the inhibition of auto oxidation of linoleic acid in aqueous alcohol system and by DPPH method along with antibacterial activity against selected organisms studied by disc diffusion method were reported. Keywords : DPPH, antioxidants, Curcuminoids, mango ginger and Curcuma amada _____________________________________________________________________________________________ INTRODUCTION The plant and plant products have enriched human civilization since time immemorial. Over the centuries humans have relied on plants for basic needs such as food, clothing, and shelter and medicine. Besides functioning as the energy source for animals, they provide raw materials for many phytochemical based industries such as pharmaceutical, textile, perfumery, flavour and food industries. The Indian subcontinent is well known for its rich heritage of herbs and spices. With different climates in different parts of the country, India produces a variety of spices and herbs, many of which are native to the subcontinent, while others were imported from similar climates and have since been cultivated locally for centuries. -

Mango Ginger (Curcuma Amada Roxb.) – a Promising Spice for Phytochemicals and Biological Activities
Review Mango ginger (Curcuma amada Roxb.) – A promising spice for phytochemicals and biological activities 1, 2 1 RS POLICEGOUDRA *, SM ARADHYA and L SINGH 1Department of Biotechnology, Defence Research Laboratory, Tezpur 784 001, India 2Fruit and Vegetable technology Department, Central Food Technological Research Institute, Mysore 570 020, India *Corresponding author (Fax, +91-3712-258534; Email, [email protected]) Mango ginger (Curcuma amada Roxb.) is a unique spice having morphological resemblance with ginger but imparts a raw mango flavour. The main use of mango ginger rhizome is in the manufacture of pickles and culinary preparations. Ayurveda and Unani medicinal systems have given much importance to mango ginger as an appetizer, alexteric, antipyretic, aphrodisiac, diuretic, emollient, expectorant and laxative and to cure biliousness, itching, skin diseases, bronchitis, asthma, hiccough and inflammation due to injuries. The biological activities of mango ginger include antioxidant activity, antibacterial activity, antifungal activity, anti-inflammatory activity, platelet aggregation inhibitory activity, cytotoxicity, antiallergic activity, hypotriglyceridemic activity, brine-shrimp lethal activity, enterokinase inhibitory activity, CNS depressant and analgesic activity. The major chemical components include starch, phenolic acids, volatile oils, curcuminoids and terpenoids like difurocumenonol, amadannulen and amadaldehyde. This article brings to light the major active components present in C. amada along with their biological activities that may be important from the pharmacological point of view. [Policegoudra RS, Aradhya SM and Singh L 2011 Mango ginger (Curcuma amada Roxb.) – A promising spice for phytochemicals and biological activities. J. Biosci. 36 739–748] DOI 10.1007/s12038-011-9106-1 1. Introduction carried out on Curcuma longa (turmeric) and Zingber offcinale (ginger), but Curcuma amada (mango ginger) is an untapped Several epidemiological studies established a link between medicinal plant of the ginger family.