Flores Vivar S.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Read Book Chefs Guide to Herbs and Spices
CHEFS GUIDE TO HERBS AND SPICES: REFERENCE GUIDE PDF, EPUB, EBOOK Inc. Barcharts | 4 pages | 23 Feb 2006 | Barcharts, Inc | 9781423201823 | English | Boca Raton, FL, United States Chefs Guide to Herbs and Spices: Reference Guide PDF Book I also love it with lentils and in my chicken salad with apples. Cinnamon is beloved in both sweet and savory dishes around the world and can be used whole as sticks or ground. Email Required. They do tend to go stale and are not as pure as fresh ones so make sure they are green and strongly aromatic when you crush them. Read more about garlic here. Ginger powder is the dried and ground root of the flowering tropical plant Zingiber officinale, and has a milder and slightly sweeter taste than that of fresh ginger root. I never use parsley in dried form. Shred it and add it to a white sauce with mustard. From European mountains , but also moorland and heaths. Shrimp Alfredo is exquisite — juicy shrimp in a cheese sauce serves with pasta. It is widely used in Indian cuisine and is woody and pungent. The results is a lovely, sweetly smoky and lightly spiced flavor often found in spicy sausages like chorizo or salami, and paella. They also last for a long time in the fridge. Anise is the dried seed of an aromatic flowering plant, Pimpinella anisum , in the Apiaceae family that is native to the Levant, or eastern Mediterranean region, and into Southwest Asia. Cinnamon has a subtle, sweet, and complex flavor with floral and clove notes. -

Approved Plant List 10/04/12
FLORIDA The best time to plant a tree is 20 years ago, the second best time to plant a tree is today. City of Sunrise Approved Plant List 10/04/12 Appendix A 10/4/12 APPROVED PLANT LIST FOR SINGLE FAMILY HOMES SG xx Slow Growing “xx” = minimum height in Small Mature tree height of less than 20 feet at time of planting feet OH Trees adjacent to overhead power lines Medium Mature tree height of between 21 – 40 feet U Trees within Utility Easements Large Mature tree height greater than 41 N Not acceptable for use as a replacement feet * Native Florida Species Varies Mature tree height depends on variety Mature size information based on Betrock’s Florida Landscape Plants Published 2001 GROUP “A” TREES Common Name Botanical Name Uses Mature Tree Size Avocado Persea Americana L Bahama Strongbark Bourreria orata * U, SG 6 S Bald Cypress Taxodium distichum * L Black Olive Shady Bucida buceras ‘Shady Lady’ L Lady Black Olive Bucida buceras L Brazil Beautyleaf Calophyllum brasiliense L Blolly Guapira discolor* M Bridalveil Tree Caesalpinia granadillo M Bulnesia Bulnesia arboria M Cinnecord Acacia choriophylla * U, SG 6 S Group ‘A’ Plant List for Single Family Homes Common Name Botanical Name Uses Mature Tree Size Citrus: Lemon, Citrus spp. OH S (except orange, Lime ect. Grapefruit) Citrus: Grapefruit Citrus paradisi M Trees Copperpod Peltophorum pterocarpum L Fiddlewood Citharexylum fruticosum * U, SG 8 S Floss Silk Tree Chorisia speciosa L Golden – Shower Cassia fistula L Green Buttonwood Conocarpus erectus * L Gumbo Limbo Bursera simaruba * L -

Pharmacological Importance of Kaempferia Galanga (Zingiberaceae): a Mini Review
International Journal of Research in Pharmacy and Pharmaceutical Sciences International Journal of Research in Pharmacy and Pharmaceutical Sciences ISSN: 2455-698X Impact Factor: RJIF 5.22 www.pharmacyjournal.in Volume 3; Issue 3; May 2018; Page No. 32-39 Pharmacological importance of Kaempferia galanga (Zingiberaceae): A mini review Hosne Jahan Shetu1, Kaniz Taskina Trisha2, Shishir Ahmed Sikta3, Raihanatul Anwar4, Sadman Sakib Bin Rashed5, Pritesh Ranjan Dash6* 1, 2, 3 Department of Pharmaceutical Sciences, North South University, Dhaka, Bangladesh 4, 5 Department of Pharmacy, BRAC University, Mohakhali, Dhaka, Bangladesh 6 Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh Abstract Kaempferia galanga L. belonging to the family Zingiberaceae is an endangered medicinal plant with potent medicinal activities. The leaves, rhizome and root tubers of the plant possess a number of medicinal applications. The plant is economically important and is over exploited to the extent that there is always scarcity of propagating material (rhizomes) which is the consumable part too. The present review provides broad information of Kaempferia galanga throwing light on its current status, ethnobotany, phytochemistry and pharmacology. Extracts of Kaempferia galanga have anti-inflammatory, analgesic, anti-diarrheal, anti- bacterial, sedative, cytotoxic, insecticidal and anthelmintic properties which are reported here. Keywords: Kaempferia galanga, zingiberaceae, phytochemistry, pharmacological activity Introduction spite of the variety of useful pharmacological properties it Kaempferia galanga Linn., commonly known as Cekor, possess. Therefore, the importance of the plant K. galanga as Ekangi, Kencur or aromatic ginger is a stem less herb in a medicinal plant is to be documented and presented to the Zingiberaceae family. The plant is native to tropical Asia mass of people. -
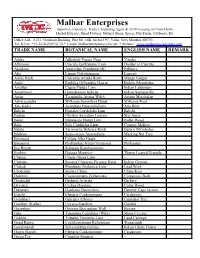
Trade Name Botanical Name English Name Remark
Malhar Enterprises Importers, Exporters, Traders, Indenting Agent & All Processing in Crude Herbs, Herbal Extracts, Dried Flowers, Natural Gums, Spices, Dry Fruits, Oil Seeds, Etc Office Add.: 2/233, Grohitam Building, Plot No. 14B, Sector 19C, Vashi, Navi Mumbai 400705. Tel. & Fax: +91-22-41236974/ 76 * E-mail: [email protected] * Website : www.malharent.tradeindia.com TRADE NAME BOTANICAL NAME ENGLISH NAME REMARK Adusa Adhatoda Vasica Nees Vasaka Aftimoon Cuscuta Epithymum Linn Dodder or Cuscuta Akarkara Anacyclus Pyrethrum DC Pellitory Alsi Linum Usitatissimum Linseed Amba Haldi Curcuma Amada Roxb Mango Ginger Amla Emblica Officinalis Gaertn Emblic Myrobalan Amaltas Cassia Fistula Linn Indian Laburnum Anantmool Hemidesmus Indicus Indian Sarsaparilla Arjun Terminalia Arjuna W&A Arjuna Myrobalan Ashwagandha Withania Somnifera Dunal Withania Root Atis Kadvi Aconitum Heterophyllum Atis Root Babchi Psoralea Corylifolia Linn Babchi Badian Illicium Anisatum Loureio Star Anise Bakul Mimusops Elengi Linn Bullet Wood Bala Sida Cordifolia Linn Country Mallow Behda Terminalia Belerica Roxb Beleric Myrobalan Bhilawa Semecarpus Anacardium Marking Nut Tree Bhringraj Eclipta Alba Hassk -- Bhuiamla Phyllanthus Niruri/ Fraternus Phyllantus Big Ringni Solanum Kanthocarpum -- Brahmi Bacopa Monnieri Thyme Leaved Gratiola Chaksu Cassia Absus Linn -- Chiraita Swertia Chirayita Fleming Karst Indian Gentian Chitrak Plumbago Zeylanica Linn. Lead Wort Chobchini Smilax China China Root Dalchini Cinnamomum Zeylanicum Cinnamom Bark Daruhaldi Berberis Aristata -

Therapeutic Effects of Bossenbergia Rotunda
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2015): 78.96 | Impact Factor (2015): 6.391 Therapeutic Effects of Bossenbergia rotunda S. Aishwarya Bachelor of Dental Surgery, Saveetha Dental College and Hospitals Abstract: Boesenbergia rotunda (L.) (Fingerroot), formerly known as Boesenbergia or Kaempferiapandurata (Roxb). Schltr. (Zingiberaceae), is distributed in south-east Asian countries, such as Indonesia, Malaysia and Thailand. The rhizomes of this plant have been used for the treatment of peptic ulcer, as well as colic, oral diseases, urinary disorders, dysentery and inflammation. As people have started to focus more on natural plants species for their curative properties. B. rotunda is a native ingredient in many Asian countries and is used as a condiment in food. It is also used as traditional medicine to treat several illnesses, consumed as traditional tonic especially after childbirth, beauty aid for teenage girls, and as a leukorrhea preventive remedy for women. Its fresh rhizomes are also used to treat inflammatory diseases, in addition to being used as an antifungal, antiparasitic, and aphrodisiac among Thai folks. Moreover, AIDS patients self-medicate themselves with B. rotunda to cure the infection. With the advancement in technology, the ethnomedicinal usages of herbal plants can be explained through in vitro and in vivo studies to prove the activities of the plant extracts. The current state of research on B. rotunda clearly shows that the isolated bioactive compounds have high potential in treating many diseases. Keywords: Zingerberaceae, anti fungal, anti parasitic, Chalcones, flavonoids. 1. Introduction panduratin derivative are prenylated flavonoids from B. pandurata that showed broad range of biological activities, Boesenbergia rotunda is a ginger species that grows in such as strong antibacterial acitivity9-11, anti- inflammatory Southeast Asia, India, Sri Lanka, and Southern China. -

Micropropagation-An in Vitro Technique for the Conservation of Alpinia Galanga
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2014, 5(3):259-263 ISSN: 0976-8610 CODEN (USA): AASRFC Micropropagation-an in vitro technique for the conservation of Alpinia galanga Nongmaithem M. Singh 1, Lukram A. Chanu 1, Yendrembam P. Devi 1, Wahengbam R.C. Singh 2 and Heigrujam B. Singh 2 1DBT-Institutional Biotech Hub, Pettigrew College, Ukhrul, Manipur 2DBT- Institutional Biotech Hub, Deptt. of Biotechnology, S.K. Women’s College, Nambol, Manipur _____________________________________________________________________________________________ ABSTRACT This study was conducted to develop an efficient protocol for mass propagation of Alpinia galanga L. Explants from rhizome buds were cultured on Murashige and Skoog (MS) medium supplemented with 6-Benzylaminopurine (BAP) alone (0 to 5 mg/l) or a combination of BAP (0 to 5 mg/l) and indole 3-acetic acid (IAA) (0 to 2 mg/l). MS medium supplemented with a combination of 5.0 mg/l BAP and 2.0 mg/l IAA or 3.0 mg/l BAP and 0.5 mg/l IAA produced the highest mean number of shoots per explant as compared to other concentrations. The best shoot length was obtained on the medium containing 1.0 mg/l of BAP and 2.0 mg/l IAA. Thus, combined effects of BAP and IAA improved significantly the shoot growth and proliferation. MS medium supplemented with a combination of 5.0 mg/l BAP and 2 mg/l IAA gave the highest number of roots. However, longest roots per explant were obtained with 1.0 mg/l BAP alone. -

C-23 Phytochemical of Kaempferia Plant And
Proceeding of International Conference On Research, Implementation And Education Of Mathematics And Sciences 2014, Yogyakarta State University, 18-20 May 2014 C-23 PHYTOCHEMICAL OF KAEMPFERIA PLANT AND BIOPROSPECTING FOR CANCER TREATMENT Sri Atun Chemistry education Faculty of Mathematical and Natural Science, Yogyakarta State University, Jl. Colombo No. 1 Yogyakarta, Indonesia, 55281 e-mail : [email protected] ABSTRACT Kaempferia genus is perennial member of the Zingiberaceae family and is cultivated in Indonesia and other parts of Southeast Asia. Number of studies has been conducted, providing information related to Kaempferia as antioxidant; antimutagenic; and chemopreventive agent. This paper reports some isolated compounds from this plant, biological activity, and bioprospecting for cancer treatment. Keyword: Cancer treatment; Kaempferia; Zingiberaceae INTRODUCTION Kaempferia is a genus, belong to family of Zingiberaceae. This plant grows in Southeast Asia, India, Sri Lanka, Indonesia, and Southem China. Kaempferia genus sinonim with Boesenbergia genus by Baker. This plant has 8 different botanical names which are Boesenbergia cochinchinensis (Gagnep.) Loes., Boesenbergia pandurata (Roxb.) Schltr., Curcuma rotunda L., Gastrochilus panduratus (Roxb.) Ridl., Gastrochilus rotundus (L.) Alston, Kaempferia cochinchinensis Gagnep., Kaempferia ovate Roscoe, Kaempferia galanga, Kaempferia rotunda, and Kaempferia pandurata Roxb nonetheless it is currently known as Boesenbergia rotunda (L.)Mansf (Tan Eng-Chong, et. al, 2012). The plants grown naturally in damp, shaded parts of the lowland or on hill slopes, as scattered plants or thickets. Economically important species among the plant families, the Zingiberaceae, which are perennial rhizomatous herbs, contain volatile oil and other important compounds of enormous medicinal values (Singh C.B., 2013). Phytochemical and biologycal activities of some species of Kaempferia Phytochemical and biologycal some species of plants of the genus Kaempferia reported by many researchers, among others: 1. -

In Vitro Antimicrobial Evaluation of Kaempferia Galanga L. Rhizome Extract Kochuthressia K
AMERICAN JOURNAL BIOTECHNOLOGY AND MOLECULAR SCIENCES ISSN Print: 2159-3698, ISSN Online: 2159-3701, doi:10.5251/ajbms.2012.2.1.1.5 © 2012, ScienceHuβ, http://www.scihub.org/AJBMS In vitro antimicrobial evaluation of Kaempferia galanga L. rhizome extract Kochuthressia K. P.1 S.John Britto2, Jaseentha M.O1 and Rini Raphael3 1Dept of Botany , Carmel College, Mala, Trissur-680732 2The Rapinat Herbarium and Centre for Molecular Systematics St.Joseph’s College (Autonomous), Tiruchirappalli-620 002 3Dept of Zoology, Carmel College, Mala, Trissur-680732 ABSTRACT In the present study, antimicrobial activity of ethanol, methanol, petroleum ether, chloroform and aqueous extracts of Kaempferia galanga rhizome were screened aganist ten human pathogenic bacteria such as Staphylococcus aureus, Streptococcus faecalis, Bacillus cereus, Bacillus subtilis, Enterobacter aerogenes, Salmonella typhi, Escherichia coli, Klebsiella pneumoniae, Pesudomonas aeruginosa and Vibrio cholerae and four fungal species :Aspergillus niger, A . flavus, A.fumigatus and Candida albicans susing disc diffusion assay. All the extracts showed significant antibacterial and antifungal properties. Highest inhibition zone (21.3±0.08) was recorded for ethanolic extract against Staphylococcus aureus. Key words : Rhizome, Kaempferia galanga, antimicrobial activity, disc diffusion assay. INTRODUCTION of medicinal plants are being increasingly reported from different parts of the world (Saxena, 1999). It is Herbal medicines are gaining priorities in treating expected that plant extracts showing target sites various health ailments of diverse origins in man. other than those used by antibiotics will be active Before the inventions of the modern synthetic against drug resistant microbial pathogen medicines, man’s dependence was totally on plants. Traditional systems of plant based products have Kaempferia galanga L. -

Utilization of Garlic and Kaemfiera on the Strength of Fungi Growth in Sardine Fish 'Pedetan' (Sardinella Lemuru)
Utilization of Garlic and Kaemfiera on the Strength of Fungi Growth in Sardine Fish ‘ Pedetan ’ ( Sardinella lemuru ) Ni Made Ayu Suardi Singapurwa 1, I Putu Candra 2 {[email protected] 1, [email protected] 2} Department of Food Science and Technology, Warmadewa University Denpasar-Bali, Indonesia 12 Abstract. Sardine fish ‘ Pedetan ’ is one of the traditional foods in the area of Jembrana Regency, Bali. During the process of storing ‘ Pedetan ’, they are often damaged by fungi that pollute the Pedetan s. This study aims to determine the use of garlic and kaemfiera on the growth of fungi that can contaminate Pedetan . The results showed that the use of garlic and kaemfiera can inhibit fungal growth. The making of sardine fish sprouts using garlic spices can inhibit the growth of fungi with a inhibition zone of 21.15 mm and kaemfiera can inhibit the inhibition zone by 25.45 mm. Garlic and kaemfiera can inhibit fungal growth because they contain bioactive compounds that can be antimicrobial. Keywords: Garlic, kaemfiera, Sardine fish Pedetan 1 Introduction Pedetan is one of the traditional Balinese spicy dried fish food products processed by the community in the Jembrana Regency area of Bali Province. The community processes and extends the shelf life of the sardine by processing it into food products that can be stored longer, which is commonly referred to as Pedetan . Pedetan made from sardine, salt and spices, is dried in the sun for two to three days, then stored at room temperature [1]. Damage to dry fish can occur during storage and during distribution in marketing. -

Galangal from Laos to Inhibit Some Foodborne Pathogens, Particularly Escherichia Coli, Salmonella Enterica Serovar
Food and Applied Bioscience Journal, 2018, 6(Special Issue on Food and Applied Bioscience), 218–239 218 Antimicrobial Activities of some Herb and Spices Extracted by Hydrodistillation and Supercritical Fluid Extraction on the Growth of Escherichia coli, Salmonella Typhimurium and Staphylococcus aureus in Microbiological Media Somhak Xainhiaxang1,2, Noppol Leksawasdi1 and Tri Indrarini Wirjantoro1,* Abstract This study investigated the antimicrobial actions of Zanthoxylum limonella, neem leaves, garlic and galangal from Laos to inhibit some foodborne pathogens, particularly Escherichia coli, Salmonella enterica serovar. Typhimurium and Staphylococcus aureus. Herb extracts were obtained by hydrodistillation at 100ºC for 4 h at atmospheric pressure or by supercritical fluid extraction at 45ºC and 17 MPa for 4 h. The antimicrobial activities of the extracts were then studied against three different pathogens on microbiological media using Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and agar disc diffusion assay. The highest yield extract was determined in the Z. limonella extract obtained by hydrodistillation, which was 6.32±0.40%. In the MIC method, the Z. limonella extract from hydrodistillation and galangal extract obtained by supercritical fluid extraction at a concentration of 12.5% could inhibit all of the studied pathogens. However, it was only the Z. limonella extract produced by hydrodistillation that could kill the pathogens at the lowest concentration of 12.5%. Regarding the agar disc diffusion assay, Z. limonella extract from hydrodistillation at 100% concentration could inhibit E. coli for 15.67±1.81 mm, which was not significantly different to that of an antibiotic control of 10 g methicillin (p≥0.05). For S. -

DPPH Radical Scavenging Potential of Ginger Leaves and Rhizomes
Research Article DPPH Radical Scavenging Potential of Ginger Leaves and Rhizomes Gina Batoy Barbosa1,*, Nonita Picardal Peteros2 1Department of Chemistry, Central Mindanao University, University Town, Musuan, Bukidnon, PHILIPPINES. 2Department of Chemistry, Mindanao State University-Iligan Institute of Technology, Iligan City, PHILIPPINES. Submission Date: 15-09-2018; Revision Date: 19-11-2018; Accepted Date: 20-12-2018 Correspondence: ABSTRACT Dr. Gina Batoy Barbosa, Introduction: Department of Chemistry, Gingers, belonging to the family Zingiberaceae, are popularly known for their Central Mindanao University, beneficial uses in medicine and culinary applications. Aim: This study was conducted to evaluate University Town, Musuan, the DPPH radical scavenging activity of the leaves and rhizomes of Zingiber officinale Rosc., Bukidnon, PHILIPPINES. Curcuma longa L., and Etlingera elatior (Jack) R.M. Smith. Methods: The plant samples were collected from Bukidnon, Mindanao, Philippines. Both water and ethanolic extracts were prepared Phone no: +63-917-426- 8951 separately from its leaves and rhizomes. The extracts were subjected to the determination of Email: ginavbatoy@yahoo. DPPH radical scavenging activity relative to ascorbic acid. Results and Discussion: Leaves, in com general, had higher radical scavenging activity in water than in ethanol extracts. On the other hand, rhizomes had generally higher radical scavenging activity in ethanol than in water extracts except for E. elatior. Among the leaf extracts, E. elatior possessed the highest radical scavenging activity. In both water and ethanol, E. elatior displayed higher radical scavenging activity in its leaves that its rhizomes. Conclusion: Findings of this study suggest the potential of E. elatior leaves as source of antioxidants. Key words: Gingers, Curcuma longa, Etlingera elatior, Zingiber officinale, DPPH. -

A Review of the Literature
Pharmacogn J. 2019; 11(6)Suppl:1511-1525 A Multifaceted Journal in the field of Natural Products and Pharmacognosy Original Article www.phcogj.com Phytochemical and Pharmacological Support for the Traditional Uses of Zingiberacea Species in Suriname - A Review of the Literature Dennis RA Mans*, Meryll Djotaroeno, Priscilla Friperson, Jennifer Pawirodihardjo ABSTRACT The Zingiberacea or ginger family is a family of flowering plants comprising roughly 1,600 species of aromatic perennial herbs with creeping horizontal or tuberous rhizomes divided into about 50 genera. The Zingiberaceae are distributed throughout tropical Africa, Asia, and the Americas. Many members are economically important as spices, ornamentals, cosmetics, Dennis RA Mans*, Meryll traditional medicines, and/or ingredients of religious rituals. One of the most prominent Djotaroeno, Priscilla Friperson, characteristics of this plant family is the presence of essential oils in particularly the rhizomes Jennifer Pawirodihardjo but in some cases also the leaves and other parts of the plant. The essential oils are in general Department of Pharmacology, Faculty of made up of a variety of, among others, terpenoid and phenolic compounds with important Medical Sciences, Anton de Kom University of biological activities. The Republic of Suriname (South America) is well-known for its ethnic and Suriname, Paramaribo, SURINAME. cultural diversity as well as its extensive ethnopharmacological knowledge and unique plant Correspondence biodiversity. This paper first presents some general information on the Zingiberacea family, subsequently provides some background about Suriname and the Zingiberacea species in the Dennis RA Mans country, then extensively addresses the traditional uses of one representative of the seven Department of Pharmacology, Faculty of Medical Sciences, Anton de Kom genera in the country and provides the phytochemical and pharmacological support for these University of Suriname, Kernkampweg 6, uses, and concludes with a critical appraisal of the medicinal values of these plants.