Genus Curcuma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preliminary Investigation of Different Extracts of Curcuma Caesia for Its
Journal of Pharmacognosy and Phytochemistry 2015; 4(2): 116-120 E-ISSN: 2278-4136 P-ISSN: 2349-8234 Preliminary investigation of different extracts of JPP 2015; 4(2): 116-120 Received: 21-05-2015 Curcuma caesia for its antibacterial effects and Accepted: 23-06-2015 cytotoxic activity Suraj Shakya Nobel College, Faculty of Health Science, Suraj Shakya, Anil Giri, Pratima Chhetri, Sujana Shakya Department of Pharmacy, Kathmandu-Nepal. Abstract The present study is carried out with the different (Petroleum ether, Diethyl ether, Chloroform and Anil Giri Methanol) extracts of dried rootstocks and rhizome of Curcuma caesia. Traditional use of it as liver Nobel College, tonic, antifungal, pain reliever as well as anthelmintic inspired us to investigate the antimicrobial and Faculty of Health Science, Department of Pharmacy, cytotoxic activity of this plant. Preliminary phytochemical screening revealed the presence of alkaloids, Kathmandu-Nepal. terpenoids, flavonoids, deoxysugars as well as C-glycosides. The present study revealed that the tannin was absent in all extract of C. caesia, which may be a possible reason for not showing any antibacterial Pratima Chhetri activity. Also the brine shrimp bioassay revealed that petroleum ether extract, diethyl ether extract, Nobel College, chloroform extract and methanol extract showed LC50 value of 18.923 mcg/ml, 1.086 mcg/ml, 45.289 Faculty of Health Science, mcg/ml and 100 mcg/ml respectively and since the LC50 values less than 1000 mcg/ml indicate the Department of Pharmacy, cytotoxic property of extracts, all the extracts of the plant were biologically active. Kathmandu-Nepal. Keywords: Curcuma caesia, phytochemical screening, Antibacterial activity, cytotoxic activity. -
Thesis Final Edition
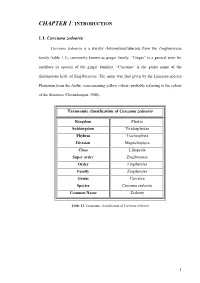
CHAPTER 1: INTRODUCTION 1.1. Curcuma zedoaria Curcuma zedoaria is a starchy rhizomatous/tuberous from the Zingiberaceae family (table 1.1), commonly known as ginger family. “Ginger” is a general term for members or species of the ginger families. “Curcuma” is the genus name of the rhizomatous herb, of Zingiberaceae. The name was first given by the Linnaeus species Plantarum from the Arabic term meaning yellow colour, probably referring to the colour of the rhizomes (Govindarajan, 1980). Taxonomic classification of Curcuma zedoaria Kingdom Plantae Subkingdom Viridaeplantae Phylum Tracheophyta Division Magnoliophyta Class Liliopsida Super order Zingiberanae Order Zingiberales Family Zingiberales Genus Curcuma Curcuma zedoaria Species Common Name Zedoary Table 1.1 Taxonomic classification of Curcuma zedoaria 1 Chapter 1 1.1.1. Description and distribution Curcuma zedoaria is locally known as “kunyit putih” or “temu putih”. It is able to grow up to one and half meters or even more. The leaves are around eighty centimetres long and they usually have a purple-brown flush along the midrib on both surfaces of the leaf. The rhizomes are frequently confused with those of Curcuma aeruginosa because both are of a similar colour (yellow). However, they can be distinguished easily by conducting a cross section on the rhizomes of the mature plants of Curcuma aeruginosa which are slightly dark purplish. In comparison, the colour of the rhizomes of Curcuma zedoaria is pale yellow or white. The rhizomes of Curcuma aeruginosa are highly aromatic due to the high amount of 1, 8-cineol as 25.20% (Ibrahim et al. 2003). Curcuma zedoaria grows mainly in the East-Asian countries including China (called Er- chu in Chinese), Vietnam, India, Bangladesh, Indonesia, Malaysia (can be found at Kuala Selangor, Teluk Intan; Perak, Labis; Johor, and Pahang) and Japan (Islam et al. -

“Joy” Winuthayanon, Bs.N., Ph.D
WIPAWEE “JOY” WINUTHAYANON, BS.N., PH.D. School of Molecular Biosciences Center for Reproductive Biology College of Veterinary Medicine Washington State University Biotechnology Life Science Building, Pullman, WA 99164 509.335.8296 | [email protected] | https://labs.wsu.edu/winuthayanon EDUCATION 2003-2009 Ph.D. Human Physiology Mahidol University, Bangkok, Thailand Doctoral Program in the Department of Physiology, Faculty of Science Evaluation and characterization for the estrogenic activity of diarylheptanoids from Curcuma comosa PI: Pawinee Piyachaturawat, Ph.D. 1998-2002 B.Sc. Nursing Science (Second Class Honors) Mahidol University, Bangkok, Thailand School of Nursing, Faculty of Medicine Ramathibodi Hospital Specialty: Nursing and Midwifery POSITIONS effective 07/2021 Associate Professor (Tenured) School of Molecular Biosciences, College of Veterinary Medicine, Washington State University (WSU), Pullman, WA Training Faculty, MARC-WSU Program (05/2021–present) Graduate Faculty, adjunct (05/2020–present) Clinical & Translational Sciences, Dept. of Veterinary Clinical Sciences, WSU Faculty Research Mentor (06/2018–present) Pacific Northwest Louis Stokes Alliance for Minority Participation (LSAMP), WSU Faculty Research Mentor (01/2016–present) Team Mentoring Program (TMP), WSU 08/2015–06/2021 Assistant Professor School of Molecular Biosciences, College of Veterinary Medicine, WSU 08/2009–07/2015 Post-doctoral Fellow National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS), Research Triangle Park (RTP), NC Reproductive & Developmental Biology Laboratory PIs: Kenneth Korach, Ph.D. and Carmen Williams, M.D. Ph.D. 03/2007–06/2008 Pre-doctoral Fellow NIH/NIEHS, Research Triangle Park, NC Laboratory of Reproduction and Developmental Toxicology PI: Kenneth Korach, Ph.D. 06/2003–01/2009 Graduate Research Assistant Department of Physiology, Faculty of Sciences, Bangkok, Thailand PI: Pawinee Piyachaturawat, Ph.D. -

Chemical Composition and Product Quality Control of Turmeric
Stephen F. Austin State University SFA ScholarWorks Faculty Publications Agriculture 2011 Chemical composition and product quality control of turmeric (Curcuma longa L.) Shiyou Li Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Wei Yuan Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Guangrui Deng Ping Wang Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Peiying Yang See next page for additional authors Follow this and additional works at: http://scholarworks.sfasu.edu/agriculture_facultypubs Part of the Natural Products Chemistry and Pharmacognosy Commons, and the Pharmaceutical Preparations Commons Tell us how this article helped you. Recommended Citation Li, Shiyou; Yuan, Wei; Deng, Guangrui; Wang, Ping; Yang, Peiying; and Aggarwal, Bharat, "Chemical composition and product quality control of turmeric (Curcuma longa L.)" (2011). Faculty Publications. Paper 1. http://scholarworks.sfasu.edu/agriculture_facultypubs/1 This Article is brought to you for free and open access by the Agriculture at SFA ScholarWorks. It has been accepted for inclusion in Faculty Publications by an authorized administrator of SFA ScholarWorks. For more information, please contact [email protected]. Authors Shiyou Li, Wei Yuan, Guangrui Deng, Ping Wang, Peiying Yang, and Bharat Aggarwal This article is available at SFA ScholarWorks: http://scholarworks.sfasu.edu/agriculture_facultypubs/1 28 Pharmaceutical Crops, 2011, 2, 28-54 Open Access Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.) ,1 1 1 1 2 3 Shiyou Li* , Wei Yuan , Guangrui Deng , Ping Wang , Peiying Yang and Bharat B. Aggarwal 1National Center for Pharmaceutical Crops, Arthur Temple College of Forestry and Agriculture, Stephen F. -
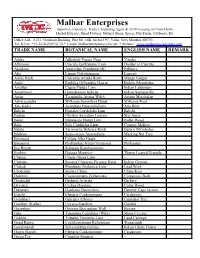
Trade Name Botanical Name English Name Remark
Malhar Enterprises Importers, Exporters, Traders, Indenting Agent & All Processing in Crude Herbs, Herbal Extracts, Dried Flowers, Natural Gums, Spices, Dry Fruits, Oil Seeds, Etc Office Add.: 2/233, Grohitam Building, Plot No. 14B, Sector 19C, Vashi, Navi Mumbai 400705. Tel. & Fax: +91-22-41236974/ 76 * E-mail: [email protected] * Website : www.malharent.tradeindia.com TRADE NAME BOTANICAL NAME ENGLISH NAME REMARK Adusa Adhatoda Vasica Nees Vasaka Aftimoon Cuscuta Epithymum Linn Dodder or Cuscuta Akarkara Anacyclus Pyrethrum DC Pellitory Alsi Linum Usitatissimum Linseed Amba Haldi Curcuma Amada Roxb Mango Ginger Amla Emblica Officinalis Gaertn Emblic Myrobalan Amaltas Cassia Fistula Linn Indian Laburnum Anantmool Hemidesmus Indicus Indian Sarsaparilla Arjun Terminalia Arjuna W&A Arjuna Myrobalan Ashwagandha Withania Somnifera Dunal Withania Root Atis Kadvi Aconitum Heterophyllum Atis Root Babchi Psoralea Corylifolia Linn Babchi Badian Illicium Anisatum Loureio Star Anise Bakul Mimusops Elengi Linn Bullet Wood Bala Sida Cordifolia Linn Country Mallow Behda Terminalia Belerica Roxb Beleric Myrobalan Bhilawa Semecarpus Anacardium Marking Nut Tree Bhringraj Eclipta Alba Hassk -- Bhuiamla Phyllanthus Niruri/ Fraternus Phyllantus Big Ringni Solanum Kanthocarpum -- Brahmi Bacopa Monnieri Thyme Leaved Gratiola Chaksu Cassia Absus Linn -- Chiraita Swertia Chirayita Fleming Karst Indian Gentian Chitrak Plumbago Zeylanica Linn. Lead Wort Chobchini Smilax China China Root Dalchini Cinnamomum Zeylanicum Cinnamom Bark Daruhaldi Berberis Aristata -

Inferring the Phylogeny and Divergence of Chinese Curcuma (Zingiberaceae) in the Hengduan Mountains of the Qinghai–Tibet Plateau by Reduced Representation Sequencing
Article Inferring the Phylogeny and Divergence of Chinese Curcuma (Zingiberaceae) in the Hengduan Mountains of the Qinghai–Tibet Plateau by Reduced Representation Sequencing Heng Liang 1,†, Jiabin Deng 2,†, Gang Gao 3, Chunbang Ding 1, Li Zhang 4, Ke Xu 5, Hong Wang 6 and Ruiwu Yang 1,* 1 College of Life Science, Sichuan Agricultural University, Yaan 625014, China; [email protected] (H.L.); [email protected] (C.D.) 2 School of Geography and Resources, Guizhou Education University, Guiyang 550018, China; [email protected] 3 College of Life Sciences and Food Engineering, Yibin University, Yibin 644000, China; [email protected] 4 College of Science, Sichuan Agricultural University, Yaan 625014, China; [email protected] 5 Sichuan Horticultural Crop Technical Extension Station, Chengdu 610041, China; [email protected] 6 Jiangsu Key Laboratory for Horticultural Crop Genetic Improvement, Institute of Pomology, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China; [email protected] * Correspondence: [email protected] † These authors have contributed equally to this work. Abstract: Clarifying the genetic relationship and divergence among Curcuma L. (Zingiberaceae) species around the world is intractable, especially among the species located in China. In this study, Citation: Liang, H.; Deng, J.; Gao, G.; Reduced Representation Sequencing (RRS), as one of the next generation sequences, has been applied Ding, C.; Zhang, L.; Xu, K.; Wang, H.; to infer large scale genotyping of major Chinese Curcuma species which present little differentiation Yang, R. Inferring the Phylogeny and of morphological characteristics and genetic traits. The 1295 high-quality SNPs (reduced-filtered Divergence of Chinese Curcuma SNPs) were chosen from 997,988 SNPs of which were detected from the cleaned 437,061 loci by (Zingiberaceae) in the Hengduan RRS to investigate the phylogeny and divergence among eight major Curcuma species locate in the Mountains of the Qinghai–Tibet Hengduan Mountains of the Qinghai–Tibet Plateau (QTP) in China. -

Antioxidant and Bactericidal Activity of Wild Turmeric Extracts
Journal of Pharmacognosy and Phytochemistry 2014; 2 (6): 89-94 ISSN 2278-4136 ISSN 2349-8234 JPP 2014; 2 (6): 89-94 Antioxidant and bactericidal activity of wild turmeric Received: 10-02-2014 Accepted: 01-03-2014 extracts Rachana S. and P. Venugopalan P. Venugopalan Department of Chemistry, Sree Neelakanta Government Sanskrit ABSTRACT College Pattambi, Palakkad, Kerala - Naturally occurring antioxidants have considerable importance in medicine and in food processing. 679 306, India. In this work, the antioxidant activity of dried rhizomes extract of the spice Curcuma aromatica (wild Email: [email protected] turmeric), a unique spice having a wide range of pharmacological and cosmetological applications is studied by the inhibition of auto oxidation of linoleic acid in aqueous alcohol system and by DPPH Rachana S. method along with antibacterial activity against selected organisms studied by disc diffusion method Department of Chemistry, Sree were reported. Neelakanta Government Sanskrit College Pattambi, Palakkad, Kerala - 679 306, India. Keywords: DPPH, Antioxidants, Curcumin, and Curcuma aromatica. Email: [email protected] 1. Introduction The plant cells are highly sophisticated chemical factories where a large number of chemicals are manufactured with great precision and ease from simple raw materials at normal temperature and pressure. Besides functioning as the energy source for animals, they provide raw materials for many phytochemical based industries such as pharmaceutical, perfumery, flavour and food industries. Plant extracts are known to exert a wide range of beneficial physiological effects which is reflected in their use in traditional medicines. Even in the modern age, exploring the different pharmacological action of medicinal plants and isolation of bioactive constituents [1] present in them constitute a major research area all over the world . -

Prioritization of Medicinal Plant for Their Development
PRIORITIZATION OF MEDICINAL PLANT FOR THEIR DEVELOPMENT Criteria for prioritization The National Medicinal Plant Board initially prioritized 32 medicinal plants at national level for their conservation and development. Recently, the list has been revised and 82 species have been included in the list. For the overall development of the medicinal plant sector in the state, there is a need to prioritize various medicinal plant species. This prioritization has to be based on different criteria such as ,(i) criteria for economic development, (ii) Prioritization to address the primary health care of the local community, (iii) medicinal plants prioritized for home and institutional garden, and (iv) prioritization of medicinal plants with conservation value. In the following section we have tried to touch upon different priorities relevant to the state. Medicinal Plants prioritized for trade for high income. The most important criterion they needs to be considered while prioritizing the species for high income is that the plants should be suitable to grow in the prevalent agroclimatic conditions of the state. The species should have high trade value. It should have consistently high demand. The collection, harvest and post harvest technology should suit to the site conditions of Meghalaya.There should have easy access to planting material and it should be comparatively easy to grow. Preference will also be given to those species which are suitable to grow in multi-tier plantations. The selected species should not get easily deteriorated on storage and continued cultivation. They should have enhanced scope for value addition either through primary processing or through secondary processing. A list of top ten prioritized species for obtaining high income through cultivation and trade is given in Table 18. -

Assessment of Turmeric (Curcuma Longa L.) Varieties for Yield and Curcumin Content
Assessment of Turmeric (Curcuma longa L.) Varieties for Yield and Curcumin Content by Shanheng Shi A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirement for the Degree of Master of Science Auburn, Alabama August 8th, 2020 Keywords: turmeric, curcumin, yield, concentration Approved by Dennis A. Shannon, Chair, Professor Emeritus of Crop, Soil and Environmental Sciences, Auburn University Kathy Lawrence, Professor of Entomology and Plant Pathology, Auburn University Alvaro Sanz-Saez, Assistant Professor of Crop, Soil and Environmental Sciences, Auburn University Wheeler G. Foshee, Associate Professor of Horticulture, Auburn University Srinivasa Mentreddy, Professor of Biological and Environmental Sciences, Alabama A&M University Abstract Turmeric (Curcuma longa L.) is a rhizomatous herbaceous perennial plant belonging to the ginger family, Zingiberaceae. Currently, more than 80% of turmeric is produced by India and turmeric products are exported to numerous countries. Other Asian countries, including China, Vietnam, Pakistan and Japan also grow significant amounts of turmeric. With the development of medicinal related research, turmeric shows huge potential impacts on cure cancer, prevent Alzheimer’s disease and treat other diseases caused by inflammation. Turmeric is a new crop in Alabama. There is little available published information related to cultivation and planting varieties of turmeric in the United States, however turmeric has been successfully grown on the Auburn University Agronomy Farm since 2006. Researchers and farmers lack information on turmeric varieties that produce high yield and high content of curcumin, which determine the final benefits from this crop. Turmeric varieties were collected from various sources and tested in field trials during 2016 through 2018. -

Synthesis and Pharmacological Screening of Novel 1,5
Vol 6, Issue 3, 2013 ISSN - 0974-2441 Review Article TURMERIC: NATURE’S PRECIOUS MEDICINE DUGGI SHRISHAIL1, HANDRAL HARISH K2, HANDRAL RAVICHANDRA3, G.TULSIANAND4, S.D. SHRUTHI 5 1Department of Plant Breeding and Genetics, College of Agriculture, Vellayani Thiruvananthapuram, Kerala, India. 2 Discipline of Oral Sciences, Faculty of Dentistry, National University of Singapore, Singapore. 3Department of Orthodontics and Dentofacial Othropaedics, P.M.N.M Dental College and Hospital, Bagalkot, Karnataka, India. 4Department of Biochemistry, A M C college, Bangalore, Karnataka, India. 5Department of Biotechnology, The Oxford college of Science, Bangalore, Karnataka, India. Email- [email protected] Received: 23 January 2013, Revised and Accepted: 18 February 2013 ABSTRACT Ethanobotany is a recent branch of natural science dealing with various aspects such as anthropology, archeology, botany, ecology, economics and medicine, religious, cultural and several other disciplines. Recently, great interest is given to studies of herbal drugs and traditional remedies are indicated worldwide and there has been an upsurge in the scientific investigations in area. Although turmeric (Curcuma longa and Curcuma aromatica Salisb.) has been described in Ayurveda, as treatment inflammatory diseases and is referred by different names in different cultures, active principle called Curcumin or diferuloylmethane, a yellow pigment present in turmeric (curry powder) has been shown to exhibit numerous activities. Extensive research over last fifty years has revealed several important functions of curcumin. The present study was aimed to review the ethanobotanical properties, pharmacognostic, phytochemical and pharmacological properties of turmeric plant. Root part of the plant are widely used by different tribal communities as turmeric have been shown to have wide spectrum of biological actions, which include anti-inflammatory, anti-diabetic, analgesic, antibacterial, anti-fungal, anti-protozoal, anti-ulcer, hypocholesteremic activities. -

Propagation of Zingiberaceae and Heliconiaceae1
14 Sociedade Brasileira de Floricultura e Plantas Ornamentais Propagation of Zingiberaceae and Heliconiaceae1 RICHARD A.CRILEY Department of Horticulture, University of Hawaii, Honolulu, Hawaii USA 96822 Increased interest in tropical cut vegetative growths will produce plants flower export in developing nations has identical to the parent. A few lesser gen- increased the demand for clean planting era bear aerial bulbil-like structures in stock. The most popular items have been bract axils. various gingers (Alpinia, Curcuma, and Heliconia) . This paper reviews seed and Seed vegetative methods of propagation for Self-incompatibility has been re- each group. Auxins such as IBA and ported in Costus (WOOD, 1992), Alpinia NAA enhanced root development on aerial purpurata (HIRANO, 1991), and Zingiber offshoots of Alpinia at the rate 500 ppm zerumbet (IKEDA & T ANA BE, 1989); while while the cytokinin, PBA, enhanced ba- other gingers set seed readily. sal shoot development at 100 ppm. Rhi- zomes of Heliconia survived treatment in The seeds of Alpinia, Etlingera, and Hedychium are borne in round or elon- 4811 C hot water for periods up to 1 hour gated capsules which split when the seeds and 5011 C up to 30 minutes in an experi- are ripe and ready for dispersai. ln some ment to determine their tolerance to tem- species a fleshy aril, bright orange or scar- pera tures for eradicating nematodes. Iet in color, covers .the seed, perhaps to Pseudostems soaks in 400 mg/LN-6- make it more attractive to birds. The seeds benzylaminopurine improved basal bud of gingers are black, about 3 mm in length break on heliconia rhizomes. -

Thai Zingiberaceae : Species Diversity and Their Uses
URL: http://www.iupac.org/symposia/proceedings/phuket97/sirirugsa.html © 1999 IUPAC Thai Zingiberaceae : Species Diversity And Their Uses Puangpen Sirirugsa Department of Biology, Faculty of Science, Prince of Songkla University, Hat Yai, Thailand Abstract: Zingiberaceae is one of the largest families of the plant kingdom. It is important natural resources that provide many useful products for food, spices, medicines, dyes, perfume and aesthetics to man. Zingiber officinale, for example, has been used for many years as spices and in traditional forms of medicine to treat a variety of diseases. Recently, scientific study has sought to reveal the bioactive compounds of the rhizome. It has been found to be effective in the treatment of thrombosis, sea sickness, migraine and rheumatism. GENERAL CHARACTERISTICS OF THE FAMILY ZINGIBERACEAE Perennial rhizomatous herbs. Leaves simple, distichous. Inflorescence terminal on the leafy shoot or on the lateral shoot. Flower delicate, ephemeral and highly modified. All parts of the plant aromatic. Fruit a capsule. HABITATS Species of the Zingiberaceae are the ground plants of the tropical forests. They mostly grow in damp and humid shady places. They are also found infrequently in secondary forest. Some species can fully expose to the sun, and grow on high elevation. DISTRIBUTION Zingiberaceae are distributed mostly in tropical and subtropical areas. The center of distribution is in SE Asia. The greatest concentration of genera and species is in the Malesian region (Indonesia, Malaysia, Singapore, Brunei, the Philippines and Papua New Guinea) *Invited lecture presented at the International Conference on Biodiversity and Bioresources: Conservation and Utilization, 23–27 November 1997, Phuket, Thailand.