Chemical Composition and Product Quality Control of Turmeric
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Technical Developments in the Use of Spices Dr David Baines Baines Food Consultancy Ltd
EUROPEAN SPICE ASSOCIATION GENERAL ASSEMBLY 2013 Technical Developments in the Use of Spices Dr David Baines Baines Food Consultancy Ltd Co-editor: Flavour Horizons TECHNICAL DEVELOPMENTS IN THE USE OF SPICES TOPICS: Recent health claims submitted to the EU for the use of spices Compounds in selected spices that have beneficial effects on health The use of spices to inhibit of carcinogen formation in cooked meats The growing use of spices in animal feeds Salt reduction using spices Interesting culinary herbs from Vietnam Recent Health Claims Submitted to the EU EU REGULATION OF HEALTH CLAIMS • The Nutrition and Health Claims Regulation, 1924/2006/EC is designed to ensure a high level of protection for consumers and legal clarity and fair competition for food business operators. • Claims must not mislead consumers; they must be, accurate, truthful, understandable and substantiated by science. • Implementation of this Regulation requires the adoption of a list of permitted health claims, based on an assessment by the European Food Safety Authority (EFSA) of the science substantiating the claimed effect and compliance with the other general and specific requirements of the Regulation. • This list of permitted health claims was adopted in May 2012 by the Commission and became binding on 14th December 2012. Food companies must comply from this date or face prosecution for misleading marketing. APPROVAL OF CLAIMS EU REGULATION OF HEALTH CLAIMS CLAIMS BY COMPONENT CLAIMS BY FUNCTION CLAIMS FOR SPICES – NOT APPROVED/ON HOLD SPICE CLAIM(S) Anise / Star Anise Respiratory Health, Digestive Health, Immune Health, Lactation Caraway Digestive Health, Immune Health, Lactation Cardamon Respiratory Health, Digestive Health, Immune Health, Kidney Health, Nervous System Health, Cardiovascular Health, Capsicum Thermogenesis, Increasing Energy Expenditure, Enhancing Loss of Calories, Body Weight Loss, Stomach Health, Reduction of Oxidative Stress, promotion of Hair Growth. -

Tips to Roast Vegetables Spice Guide
Tips to Roast Vegetables • Roast at a high oven temp- 400 to 450 degrees F • Chop vegetables in uniform size so they cook evenly • Don’t over crowd the pan, otherwise they will become soft • Roasting veggies with some oil will help them become crispier • To get the most flavor/crispier roast them on the top rack • Seasoning before putting them in the oven will add flavor • Flip veggies halfway through to ensure even cooking • When roasting multiple types of veggies, ensure they have similar cooking times. Good pairs include: Cauliflower and Broccoli cc Carrots and Broccoli Baby potatoes and Butternut Squash Onions and Bell Peppers Zucchini and Yellow Squash Asparagus and Leeks Spice Guide Table of Contents Spices by Cuisine Herbs and Spices 1 Mexican Coriander, Cumin, oregano, garlic powder, cinnamon, chili powder Herbs and Spices that Pair well with Proteins 2 Caribbean Chicken Fajita Bowl Recipe 3 All spice, nutmeg, garlic powder, cloves, cinnamon, ginger Shelf life of Herbs and Spices 4 French Nutmeg, thyme, garlic powder, rosemary, oregano, Herbs de Provence Spices by Cuisine 5 North African Tips to Roast Vegetables BP Cardamum, cinnamon, cumin, paprika, turmeric, ginger Cajun Cayenne, oregano, paprika, thyme, rosemary, bay leaves, Cajun seasoning Thai Basil, cumin, garlic, ginger, turmeric, cardamum, curry powder Mediterranean Oregano, rosemary, thyme, bay leaves, cardamum, cinnamon, cloves, coriander, basil, ginger Indian Bay leaves, cardamum, cayenne, cinnamon, coriander, cumin, ginger, nutmeg, paprika, turmeric, garam masala, curry powder Middle Eastern Bay leaves, cardamum, cinnamon, cloves, cumin, ginger, coriander, oregano, za’atar, garlic powder 5 Shelf Life of Herbs and Herbs and Spices Spices Herbs Herbs are plants that’s leaves can be used to add flavor to foods. -
Thesis Final Edition
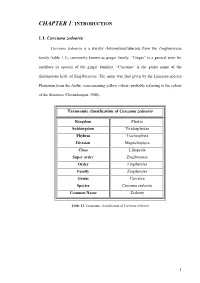
CHAPTER 1: INTRODUCTION 1.1. Curcuma zedoaria Curcuma zedoaria is a starchy rhizomatous/tuberous from the Zingiberaceae family (table 1.1), commonly known as ginger family. “Ginger” is a general term for members or species of the ginger families. “Curcuma” is the genus name of the rhizomatous herb, of Zingiberaceae. The name was first given by the Linnaeus species Plantarum from the Arabic term meaning yellow colour, probably referring to the colour of the rhizomes (Govindarajan, 1980). Taxonomic classification of Curcuma zedoaria Kingdom Plantae Subkingdom Viridaeplantae Phylum Tracheophyta Division Magnoliophyta Class Liliopsida Super order Zingiberanae Order Zingiberales Family Zingiberales Genus Curcuma Curcuma zedoaria Species Common Name Zedoary Table 1.1 Taxonomic classification of Curcuma zedoaria 1 Chapter 1 1.1.1. Description and distribution Curcuma zedoaria is locally known as “kunyit putih” or “temu putih”. It is able to grow up to one and half meters or even more. The leaves are around eighty centimetres long and they usually have a purple-brown flush along the midrib on both surfaces of the leaf. The rhizomes are frequently confused with those of Curcuma aeruginosa because both are of a similar colour (yellow). However, they can be distinguished easily by conducting a cross section on the rhizomes of the mature plants of Curcuma aeruginosa which are slightly dark purplish. In comparison, the colour of the rhizomes of Curcuma zedoaria is pale yellow or white. The rhizomes of Curcuma aeruginosa are highly aromatic due to the high amount of 1, 8-cineol as 25.20% (Ibrahim et al. 2003). Curcuma zedoaria grows mainly in the East-Asian countries including China (called Er- chu in Chinese), Vietnam, India, Bangladesh, Indonesia, Malaysia (can be found at Kuala Selangor, Teluk Intan; Perak, Labis; Johor, and Pahang) and Japan (Islam et al. -

Entomotoxicity of Xylopia Aethiopica and Aframomum Melegueta In
Volume 8, Number 4, December .2015 ISSN 1995-6673 JJBS Pages 263 - 268 Jordan Journal of Biological Sciences EntomoToxicity of Xylopia aethiopica and Aframomum melegueta in Suppressing Oviposition and Adult Emergence of Callasobruchus maculatus (Fabricus) (Coleoptera: Chrysomelidae) Infesting Stored Cowpea Seeds Jacobs M. Adesina1,3,*, Adeolu R. Jose2, Yallapa Rajashaker3 and Lawrence A. 1 Afolabi 1Department of Crop, Soil and Pest Management Technology, Rufus Giwa Polytechnic, P. M. B. 1019, Owo, Ondo State. Nigeria; 2 Department of Science Laboratory Technology, Environmental Biology Unit, Rufus Giwa Polytechnic, P. M. B. 1019, Owo, Ondo State. Nigeria; 3 Insect Bioresource Laboratory, Institute of Bioresources and Sustainable Development, Department of Biotechnology, Government of India, Takyelpat, Imphal, 795001, Manipur, India. Received: June 13, 2015 Revised: July 3, 2015 Accepted: July 19, 2015 Abstract The cowpea beetle, Callosobruchus maculatus (Fabricus) (Coleoptera: Chrysomelidae), is a major pest of stored cowpea militating against food security in developing nations. The comparative study of Xylopia aethiopica and Aframomum melegueta powder in respect to their phytochemical and insecticidal properties against C. maculatus was carried out using a Complete Randomized Design (CRD) with five treatments (0, 1.0, 1.5, 2.0 and 2.5g/20g cowpea seeds corresponding to 0.0, 0.05, 0.075, 0.1 and 0.13% v/w) replicated thrice under ambient laboratory condition (28±2°C temperature and 75±5% relative humidity). The phytochemical screening showed the presence of flavonoids, saponins, tannins, cardiac glycoside in both plants, while alkaloids was present in A. melegueta and absent in X. aethiopica. The mortality of C. maculatus increased gradually with exposure time and dosage of the plant powders. -

In Vitro Immunopharmacological Profiling of Ginger (Zingiber Officinale Roscoe)
Research Collection Doctoral Thesis In vitro immunopharmacological profiling of ginger (Zingiber officinale Roscoe) Author(s): Nievergelt, Andreas Publication Date: 2011 Permanent Link: https://doi.org/10.3929/ethz-a-006717482 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library DISS. ETH Nr. 19591 In Vitro Immunopharmacological Profiling of Ginger (Zingiber officinale Roscoe) ABHANDLUNG zur Erlangung des Titels DOKTOR DER WISSENSCHAFTEN der ETH ZÜRICH vorgelegt von Andreas Nievergelt Eidg. Dipl. Apotheker, ETH Zürich geboren am 18.12.1978 von Schleitheim, SH Angenommen auf Antrag von Prof. Dr. Karl-Heinz Altmann, Referent Prof. Dr. Jürg Gertsch, Korreferent Prof. Dr. Michael Detmar, Korreferent 2011 Table of Contents Summary 6 Zusammenfassung 7 Acknowledgements 8 List of Abbreviations 9 1. Introduction 13 1.1 Ginger (Zingiber officinale) 13 1.1.1 Origin 14 1.1.2 Description 14 1.1.3 Chemical Constituents 15 1.1.4 Traditional and Modern Pharmaceutical Use of Ginger 17 1.1.5 Reported In Vitro Effects 20 1.2 Immune System and Inflammation 23 1.2.1 Innate and Adaptive Immunity 24 1.2.2 Cytokines in Inflammation 25 1.2.3 Pattern Recognition Receptors 29 1.2.4 Toll-Like Receptors 30 1.2.5 Serotonin 1A and 3 Receptors 32 1.2.6 Phospholipases A2 33 1.2.7 MAP Kinases 36 1.2.8 Fighting Inflammation, An Ongoing Task 36 1.2.9 Inflammation Assays Using Whole Blood 38 1.3 Arabinogalactan-Proteins 39 1.3.1 Origin and Biological Function of AGPs 40 1.3.2 Effects on Animals 41 1.3.3 The ‘Immunostimulation’ Theory 42 1/188 2. -

Hyperforin Inhibits Cell Growth by Inducing Intrinsic and Extrinsic
ANTICANCER RESEARCH 37 : 161-168 (2017) doi:10.21873/anticanres.11301 Hyperforin Inhibits Cell Growth by Inducing Intrinsic and Extrinsic Apoptotic Pathways in Hepatocellular Carcinoma Cells I-TSANG CHIANG 1,2,3* , WEI-TING CHEN 4* , CHIH-WEI TSENG 5, YEN-CHUNG CHEN 2,6 , YU-CHENG KUO 7, BI-JHIH CHEN 8, MAO-CHI WENG 9, HWAI-JENG LIN 10,11# and WEI-SHU WANG 2,12,13# Departments of 1Radiation Oncology, 6Pathology, and 13 Medicine, and 2Cancer Medical Care Center, National Yang-Ming University Hospital, Yilan, Taiwan, R.O.C.; 3Department of Radiological Technology, Central Taiwan University of Science and Technology, Taichung, Taiwan, R.O.C.; 4Department of Psychiatry, Zuoying Branch of Kaohsiung Armed Forces General Hospital, Kaohsiung, Taiwan, R.O.C.; 5Division of Gastroenterology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chia-Yi, Taiwan, R.O.C.; 7Radiation Oncology, Show Chwan Memorial Hospital, Changhua, Taiwan, R.O.C.; 8Department of Laboratory Medicine, Changhua Christian Hospital, Changhua Christian Medical Foundation, Changhua, Taiwan, R.O.C.; 9Institute of Nuclear Energy Research, Atomic Energy Council, Taoyuan, Taiwan, R.O.C.; 10 Department of Internal Medicine, Division of Gastroenterology and Hepatology, College of Medicine, School of Medicine, Taipei Medical University, Taipei, Taiwan, R.O.C.; 11 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Shuang-Ho Hospital, New Taipei, Taiwan, R.O.C.; 12 National Yang-Ming University School of Medicine, Taipei, Taiwan, R.O.C. Abstract. The aim of the present study was to investigate the (c-FLIP), X-linked inhibitor of apoptosis protein (XIAP), antitumor effect and mechanism of action of hyperforin in myeloid cell leukemia 1(MCL1), and cyclin-D1] were hepatocellular carcinoma (HCC) SK-Hep1 cells in vitro. -

Inferring the Phylogeny and Divergence of Chinese Curcuma (Zingiberaceae) in the Hengduan Mountains of the Qinghai–Tibet Plateau by Reduced Representation Sequencing
Article Inferring the Phylogeny and Divergence of Chinese Curcuma (Zingiberaceae) in the Hengduan Mountains of the Qinghai–Tibet Plateau by Reduced Representation Sequencing Heng Liang 1,†, Jiabin Deng 2,†, Gang Gao 3, Chunbang Ding 1, Li Zhang 4, Ke Xu 5, Hong Wang 6 and Ruiwu Yang 1,* 1 College of Life Science, Sichuan Agricultural University, Yaan 625014, China; [email protected] (H.L.); [email protected] (C.D.) 2 School of Geography and Resources, Guizhou Education University, Guiyang 550018, China; [email protected] 3 College of Life Sciences and Food Engineering, Yibin University, Yibin 644000, China; [email protected] 4 College of Science, Sichuan Agricultural University, Yaan 625014, China; [email protected] 5 Sichuan Horticultural Crop Technical Extension Station, Chengdu 610041, China; [email protected] 6 Jiangsu Key Laboratory for Horticultural Crop Genetic Improvement, Institute of Pomology, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China; [email protected] * Correspondence: [email protected] † These authors have contributed equally to this work. Abstract: Clarifying the genetic relationship and divergence among Curcuma L. (Zingiberaceae) species around the world is intractable, especially among the species located in China. In this study, Citation: Liang, H.; Deng, J.; Gao, G.; Reduced Representation Sequencing (RRS), as one of the next generation sequences, has been applied Ding, C.; Zhang, L.; Xu, K.; Wang, H.; to infer large scale genotyping of major Chinese Curcuma species which present little differentiation Yang, R. Inferring the Phylogeny and of morphological characteristics and genetic traits. The 1295 high-quality SNPs (reduced-filtered Divergence of Chinese Curcuma SNPs) were chosen from 997,988 SNPs of which were detected from the cleaned 437,061 loci by (Zingiberaceae) in the Hengduan RRS to investigate the phylogeny and divergence among eight major Curcuma species locate in the Mountains of the Qinghai–Tibet Hengduan Mountains of the Qinghai–Tibet Plateau (QTP) in China. -

Prioritization of Medicinal Plant for Their Development
PRIORITIZATION OF MEDICINAL PLANT FOR THEIR DEVELOPMENT Criteria for prioritization The National Medicinal Plant Board initially prioritized 32 medicinal plants at national level for their conservation and development. Recently, the list has been revised and 82 species have been included in the list. For the overall development of the medicinal plant sector in the state, there is a need to prioritize various medicinal plant species. This prioritization has to be based on different criteria such as ,(i) criteria for economic development, (ii) Prioritization to address the primary health care of the local community, (iii) medicinal plants prioritized for home and institutional garden, and (iv) prioritization of medicinal plants with conservation value. In the following section we have tried to touch upon different priorities relevant to the state. Medicinal Plants prioritized for trade for high income. The most important criterion they needs to be considered while prioritizing the species for high income is that the plants should be suitable to grow in the prevalent agroclimatic conditions of the state. The species should have high trade value. It should have consistently high demand. The collection, harvest and post harvest technology should suit to the site conditions of Meghalaya.There should have easy access to planting material and it should be comparatively easy to grow. Preference will also be given to those species which are suitable to grow in multi-tier plantations. The selected species should not get easily deteriorated on storage and continued cultivation. They should have enhanced scope for value addition either through primary processing or through secondary processing. A list of top ten prioritized species for obtaining high income through cultivation and trade is given in Table 18. -

Assessment of Turmeric (Curcuma Longa L.) Varieties for Yield and Curcumin Content
Assessment of Turmeric (Curcuma longa L.) Varieties for Yield and Curcumin Content by Shanheng Shi A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirement for the Degree of Master of Science Auburn, Alabama August 8th, 2020 Keywords: turmeric, curcumin, yield, concentration Approved by Dennis A. Shannon, Chair, Professor Emeritus of Crop, Soil and Environmental Sciences, Auburn University Kathy Lawrence, Professor of Entomology and Plant Pathology, Auburn University Alvaro Sanz-Saez, Assistant Professor of Crop, Soil and Environmental Sciences, Auburn University Wheeler G. Foshee, Associate Professor of Horticulture, Auburn University Srinivasa Mentreddy, Professor of Biological and Environmental Sciences, Alabama A&M University Abstract Turmeric (Curcuma longa L.) is a rhizomatous herbaceous perennial plant belonging to the ginger family, Zingiberaceae. Currently, more than 80% of turmeric is produced by India and turmeric products are exported to numerous countries. Other Asian countries, including China, Vietnam, Pakistan and Japan also grow significant amounts of turmeric. With the development of medicinal related research, turmeric shows huge potential impacts on cure cancer, prevent Alzheimer’s disease and treat other diseases caused by inflammation. Turmeric is a new crop in Alabama. There is little available published information related to cultivation and planting varieties of turmeric in the United States, however turmeric has been successfully grown on the Auburn University Agronomy Farm since 2006. Researchers and farmers lack information on turmeric varieties that produce high yield and high content of curcumin, which determine the final benefits from this crop. Turmeric varieties were collected from various sources and tested in field trials during 2016 through 2018. -

Micropropagation and Antimicrobial Activity of Curcuma Aromatica Salisb., a Threatened Aromatic Medicinal Plant
Turkish Journal of Biology Turk J Biol (2013) 37: 698-708 http://journals.tubitak.gov.tr/biology/ © TÜBİTAK Research Article doi:10.3906/biy-1212-11 Micropropagation and antimicrobial activity of Curcuma aromatica Salisb., a threatened aromatic medicinal plant 1,5 2 3 Shamima Akhtar SHARMIN , Mohammad Jahangir ALAM , Md. Mominul Islam SHEIKH , 4 5 6 Rashed ZAMAN , Muhammad KHALEKUZZAMAN , Sanjoy Chandra MONDAL , 7 6 1, Mohammad Anwarul HAQUE , Mohammad Firoz ALAM , Iftekhar ALAM * 1 Division of Applied Life Sciences (BK21 Program), College of Agriculture and Life Science, Gyeongsang National University, Jinju, Republic of Korea 2 Department of Bioscience (Integrated Bioscience Section), Graduate School of Science and Technology, Shizuoka University, Shizuoka, Japan 3 Division of Environmental Forest Sciences, College of Agriculture and Life Science, Gyeongsang National University, Jinju, Republic of Korea 4 Department of Animal Science, Institute of Agricultural Science and Technology, College of Agriculture and Life Science, Chonnam National University, Gwangju, Republic of Korea 5 Department of Genetic Engineering and Biotechnology, University of Rajshahi, Rajshahi, Bangladesh 6 Department of Botany, University of Rajshahi, Rajshahi, Bangladesh 7 Department of Biotechnology and Genetic Engineering, Islamic University, Kushtia, Bangladesh Received: 06.12.2012 Accepted: 14.06.2013 Published Online: 08.10.2013 Printed: 04.11.2013 Abstract: A rapid and improved micropropagation protocol was developed for Curcuma aromatica, a threatened aromatic medicinal plant, using rhizome sprout as the explant. Stepwise optimization of different plant growth regulators, carbon sources, and basal media was adopted to establish an efficient micropropagation protocol. When cytokinins, such as benzyl amino purine (BAP) or 6-(α,α- dimethylallylamino)-purine (2iP), were used either singly or in combination with naphthalene acetic acid (NAA) for shoot induction and multiplication, a single use of BAP was the most effective. -

Periodic Table of Herbs 'N Spices
Periodic Table of Herbs 'N Spices 11HH 1 H 2 HeHe Element Proton Element Symbol Number Chaste Tree Chile (Vitex agnus-castus) (Capsicum frutescens et al.) Hemptree, Agnus Cayenne pepper, Chili castus, Abraham's balm 118Uuo Red pepper 33LiLi 44 Be 5 B B 66 C 7 N 7N 88O O 99 F 1010 Ne Ne Picture Bear’s Garlic Boldo leaves Ceylon Cinnamon Oregano Lime (Allium ursinum) (Peumus boldus) (Cinnamomum zeylanicum) Nutmeg Origanum vulgare Fenugreek Lemon (Citrus aurantifolia) Ramson, Wild garlic Boldina, Baldina Sri Lanka cinnamon (Myristica fragrans) Oregan, Wild marjoram (Trigonella foenum-graecum) (Citrus limon) 11 Na Na 1212 Mg Mg 1313 Al Al 1414 Si Si 1515 P P 16 S S 1717 Cl Cl 1818 Ar Ar Common Name Scientific Name Nasturtium Alternate name(s) Allspice Sichuan Pepper et al. Grains of Paradise (Tropaeolum majus) (Pimenta dioica) (Zanthoxylum spp.) Perilla (Aframomum melegueta) Common nasturtium, Jamaica pepper, Myrtle Anise pepper, Chinese (Perilla frutescens) Guinea grains, Garden nasturtium, Mugwort pepper, Pimento, pepper, Japanese Beefsteak plant, Chinese Savory Cloves Melegueta pepper, Indian cress, Nasturtium (Artemisia vulgaris) Newspice pepper, et al. Basil, Wild sesame (Satureja hortensis) (Syzygium aromaticum) Alligator pepper 1919 K K 20 Ca Ca 2121 Sc Sc 2222 Ti Ti 23 V V 24 Cr Cr 2525 Mn Mn 2626 Fe Fe 2727 Co Co 2828 Ni Ni 29 Cu Cu 3030 Zn Zn 31 Ga Ga 3232 Ge Ge 3333As As 34 Se Se 3535 Br Br 36 Kr Kr Cassia Paprika Caraway (Cinnamomum cassia) Asafetida Coriander Nigella Cumin Gale Borage Kaffir Lime (Capsicum annuum) (Carum carvi) -

(Crocus Sativus L.) and / Or Turmeric (Curcuma Longa L.) Extracts on D-Galactose Deleterious Brain Effects in Rats
Journal of Pharmaceutical Research International 33(33B): 33-57, 2021; Article no.JPRI.70003 ISSN: 2456-9119 (Past name: British Journal of Pharmaceutical Research, Past ISSN: 2231-2919, NLM ID: 101631759) Comparative Study between Saffron (Crocus sativus L.) and / or Turmeric (Curcuma longa L.) Extracts on D-galactose Deleterious Brain Effects in Rats Fatma Hussien Ahmed1, Kout Elkoloub Baker1 and Alyae M. S. Gabal1* 1Biochemistry and Nutrition Department, Faculty of Women for Arts, Science and Education, Ain Shams University, Cairo, Egypt. Authors’ contributions This work was carried out in collaboration among all authors. Author KEB designed the study and revised the manuscript. Author FHA managed the analyses of the study, performed the statistical analysis and managed the literature searches. Author AMSG designed the study, wrote the protocol, managed the literature searches, wrote the first draft of the manuscript and performed the statistical analysis. All authors read and approved the final manuscript. Article Information DOI:10.9734/JPRI/2021/v33i33B31796 Editor(s): (1) Dr. Takashi Ikeno, National Center of Neurology and Psychiatry, Japan. Reviewers: (1) Kushagra Dubey, Smriti College of Pharmaceutical Education, India. (2) M. K. Mohan Maruga Raja, Parul University, India. Complete Peer review History: http://www.sdiarticle4.com/review-history/70003 Received 14 April 2021 Original Research Article Accepted 18 June 2021 Published 28 June 2021 ABSTRACT Aims: This study was designed to investigate the active chemical constituents and antioxidant capacities of saffron stigmas and turmeric rhizomes ethanolic extracts (ESE and ETE) respectively. D- galactose deleterious brain effects as well as the role of ESE and ETE supplementation against D-galactose intoxication were evaluated on male rat’s brain.