Morphological Facilitators for Vocal Learning Explored Through the Syrinx Anatomy of A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lista Roja De Las Aves Del Uruguay 1
Lista Roja de las Aves del Uruguay 1 Lista Roja de las Aves del Uruguay Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Adrián B. Azpiroz, Laboratorio de Genética de la Conservación, Instituto de Investigaciones Biológicas Clemente Estable, Av. Italia 3318 (CP 11600), Montevideo ([email protected]). Matilde Alfaro, Asociación Averaves & Facultad de Ciencias, Universidad de la República, Iguá 4225 (CP 11400), Montevideo ([email protected]). Sebastián Jiménez, Proyecto Albatros y Petreles-Uruguay, Centro de Investigación y Conservación Marina (CICMAR), Avenida Giannattasio Km 30.5. (CP 15008) Canelones, Uruguay; Laboratorio de Recursos Pelágicos, Dirección Nacional de Recursos Acuáticos, Constituyente 1497 (CP 11200), Montevideo ([email protected]). Cita sugerida: Azpiroz, A.B., M. Alfaro y S. Jiménez. 2012. Lista Roja de las Aves del Uruguay. Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Dirección Nacional de Medio Ambiente, Montevideo. Descargo de responsabilidad El contenido de esta publicación es responsabilidad de los autores y no refleja necesariamente las opiniones o políticas de la DINAMA ni de las organizaciones auspiciantes y no comprometen a estas instituciones. Las denominaciones empleadas y la forma en que aparecen los datos no implica de parte de DINAMA, ni de las organizaciones auspiciantes o de los autores, juicio alguno sobre la condición jurídica de países, territorios, ciudades, personas, organizaciones, zonas o de sus autoridades, ni sobre la delimitación de sus fronteras o límites. -

Systematic Relationships and Biogeography of the Tracheophone Suboscines (Aves: Passeriformes)
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 23 (2002) 499–512 www.academicpress.com Systematic relationships and biogeography of the tracheophone suboscines (Aves: Passeriformes) Martin Irestedt,a,b,* Jon Fjeldsaa,c Ulf S. Johansson,a,b and Per G.P. Ericsona a Department of Vertebrate Zoology and Molecular Systematics Laboratory, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden b Department of Zoology, University of Stockholm, SE-106 91 Stockholm, Sweden c Zoological Museum, University of Copenhagen, Copenhagen, Denmark Received 29 August 2001; received in revised form 17 January 2002 Abstract Based on their highly specialized ‘‘tracheophone’’ syrinx, the avian families Furnariidae (ovenbirds), Dendrocolaptidae (woodcreepers), Formicariidae (ground antbirds), Thamnophilidae (typical antbirds), Rhinocryptidae (tapaculos), and Conop- ophagidae (gnateaters) have long been recognized to constitute a monophyletic group of suboscine passerines. However, the monophyly of these families have been contested and their interrelationships are poorly understood, and this constrains the pos- sibilities for interpreting adaptive tendencies in this very diverse group. In this study we present a higher-level phylogeny and classification for the tracheophone birds based on phylogenetic analyses of sequence data obtained from 32 ingroup taxa. Both mitochondrial (cytochrome b) and nuclear genes (c-myc, RAG-1, and myoglobin) have been sequenced, and more than 3000 bp were subjected to parsimony and maximum-likelihood analyses. The phylogenetic signals in the mitochondrial and nuclear genes were compared and found to be very similar. The results from the analysis of the combined dataset (all genes, but with transitions at third codon positions in the cytochrome b excluded) partly corroborate previous phylogenetic hypotheses, but several novel arrangements were also suggested. -

REGUA Bird List July 2020.Xlsx
Birds of REGUA/Aves da REGUA Updated July 2020. The taxonomy and nomenclature follows the Comitê Brasileiro de Registros Ornitológicos (CBRO), Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee, updated June 2015 - based on the checklist of the South American Classification Committee (SACC). Atualizado julho de 2020. A taxonomia e nomenclatura seguem o Comitê Brasileiro de Registros Ornitológicos (CBRO), Lista anotada das aves do Brasil pelo Comitê Brasileiro de Registros Ornitológicos, atualizada em junho de 2015 - fundamentada na lista do Comitê de Classificação da América do Sul (SACC). -

Guia Para Observação Das Aves Do Parque Nacional De Brasília
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/234145690 Guia para observação das aves do Parque Nacional de Brasília Book · January 2011 CITATIONS READS 0 629 4 authors, including: Mieko Kanegae Fernando Lima Favaro Federal University of Rio de Janeiro Instituto Chico Mendes de Conservação da Bi… 7 PUBLICATIONS 74 CITATIONS 17 PUBLICATIONS 69 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Fernando Lima Favaro on 28 May 2014. The user has requested enhancement of the downloaded file. Brasília - 2011 GUIA PARA OBSERVAÇÃO DAS AVES DO PARQUE NACIONAL DE BRASÍLIA Aílton C. de Oliveira Mieko Ferreira Kanegae Marina Faria do Amaral Fernando de Lima Favaro Fotografia de Aves Marcelo Pontes Monteiro Nélio dos Santos Paulo André Lima Borges Brasília, 2011 GUIA PARA OBSERVAÇÃO DAS AVES DO APRESENTAÇÃO PARQUE NACIONAL DE BRASÍLIA É com grande satisfação que apresento o Guia para Observação REPÚblica FEDERATiva DO BRASIL das Aves do Parque Nacional de Brasília, o qual representa um importante instrumento auxiliar para os observadores de aves que frequentam ou que Presidente frequentarão o Parque, para fins de lazer (birdwatching), pesquisas científicas, Dilma Roussef treinamentos ou em atividades de educação ambiental. Este é mais um resultado do trabalho do Centro Nacional de Pesquisa e Vice-Presidente Conservação de Aves Silvestres - CEMAVE, unidade descentralizada do Instituto Michel Temer Chico Mendes de Conservação da Biodiversidade (ICMBio) e vinculada à Diretoria de Conservação da Biodiversidade. O Centro tem como missão Ministério do Meio Ambiente - MMA subsidiar a conservação das aves brasileiras e dos ambientes dos quais elas Izabella Mônica Vieira Teixeira dependem. -

Passerines: Perching Birds
3.9 Orders 9: Passerines – perching birds - Atlas of Birds uncorrected proofs 3.9 Atlas of Birds - Uncorrected proofs Copyrighted Material Passerines: Perching Birds he Passeriformes is by far the largest order of birds, comprising close to 6,000 P Size of order Cardinal virtues Insect-eating voyager Multi-purpose passerine Tspecies. Known loosely as “perching birds”, its members differ from other Number of species in order The Northern or Common Cardinal (Cardinalis cardinalis) The Common Redstart (Phoenicurus phoenicurus) was The Common Magpie (Pica pica) belongs to the crow family orders in various fine anatomical details, and are themselves divided into suborders. Percentage of total bird species belongs to the cardinal family (Cardinalidae) of passerines. once thought to be a member of the thrush family (Corvidae), which includes many of the larger passerines. In simple terms, however, and with a few exceptions, passerines can be described Like the various tanagers, grosbeaks and other members (Turdidae), but is now known to belong to the Old World Like many crows, it is a generalist, with a robust bill adapted of this diverse group, it has a thick, strong bill adapted to flycatchers (Muscicapidae). Its narrow bill is adapted to to feeding on anything from small animals to eggs, carrion, as small birds that sing. feeding on seeds and fruit. Males, from whose vivid red eating insects, and like many insect-eaters that breed in insects, and grain. Crows are among the most intelligent of The word passerine derives from the Latin passer, for sparrow, and indeed a sparrow plumage the family is named, are much more colourful northern Europe and Asia, this species migrates to Sub- birds, and this species is the only non-mammal ever to have is a typical passerine. -

Phylogeny, Biogeography, and Evolution of the Broadbills (Eurylaimidae) and Asities (Philepittidae) Based on Morphology
The Auk 110(2):304-324, 1993 PHYLOGENY, BIOGEOGRAPHY, AND EVOLUTION OF THE BROADBILLS (EURYLAIMIDAE) AND ASITIES (PHILEPITTIDAE) BASED ON MORPHOLOGY RICHARD O. ?RUM Museumof Natural Historyand Departmentof Systematicsand Ecology, Universityof Kansas,Lawrence, Kansas 66045, USA ABsTRACT.--Phylogeneticanalysis of syringealmorphology and two osteologicalcharacters indicatesthat the broadbills (Eurylaimidae)are not monophyletic,but consistof four clades with successivelycloser relationships to the Madagascanasities (Philepittidae). An analysis of thesedata combined with hindlimb myologycharacters described by Raikow(1987) yields the sameresult. The sistergroup to Philepittaand Neodrepanisis the African broadbill Pseu- docalyptomena.The sistergroup to this cladeincludes all of the Asian broadbills,except the monophyleticgenus Calyptomena. The African genusSmithornis is the sistergroup to all other broadbillsand asities.A biogeographicanalysis indicates that the Madagascanendemics share a most-recentbiogeographic connection with the central African genusPseudocalyptomena. Phylogeneticassociations between transitions in bill morphologyand diet indicatethat bill morphologieshave evolved both in associationwith evolution of frugivory and nectarivory, and in apparentresponse to intrinsicfactors within the contextof frugivorousand insectiv- orousdiets. A phylogeneticclassification of the broadbillsand asitiesis proposedin which all broadbillsand asitiesare placed in five subfamiliesof the Eurylaimidae,and the separate family Philepittidae is abandoned.Received -

Assessing Bird Species Popularity with Culturomics
Familiarity breeds content: assessing bird species popularity with culturomics Ricardo A. Correia1,2, Paul R. Jepson2, Ana C. M. Malhado1 and Richard J. Ladle1,2 1 Institute of Biological and Health Sciences, Federal University of Alagoas, Maceio´, Alagoas, Brazil 2 School of Geography and the Environment, University of Oxford, Oxford, United Kingdom ABSTRACT Understanding public perceptions of biodiversity is essential to ensure continued support for conservation efforts. Despite this, insights remain scarce at broader spatial scales, mostly due to a lack of adequate methods for their assessment. The emergence of new technologies with global reach and high levels of participation provide exciting new opportunities to study the public visibility of biodiversity and the factors that drive it. Here, we use a measure of internet saliency to assess the national and international visibility of species within four taxa of Brazilian birds (toucans, hummingbirds, parrots and woodpeckers), and evaluate how much of this visibility can be explained by factors associated with familiarity, aesthetic appeal and conservation interest. Our results strongly indicate that familiarity (human population within the range of a species) is the most important factor driving internet saliency within Brazil, while aesthetic appeal (body size) best explains variation in international saliency. Endemism and conservation status of a species had small, but often negative, effects on either metric of internet saliency. While further studies are needed to evaluate the relationship between internet content and the cultural visibility of different species, our results strongly indicate that internet saliency can be considered as a broad proxy of cultural interest. Submitted 4 December 2015 Subjects Biodiversity, Conservation biology, Computational science, Coupled natural and human Accepted 2 February 2016 systems Published 25 February 2016 Keywords Birds, Public perception, Biodiversity, Culturalness, Internet salience, Culturomics, Corresponding author Conservation Ricardo A. -

Biodiversity in Sub-Saharan Africa and Its Islands Conservation, Management and Sustainable Use
Biodiversity in Sub-Saharan Africa and its Islands Conservation, Management and Sustainable Use Occasional Papers of the IUCN Species Survival Commission No. 6 IUCN - The World Conservation Union IUCN Species Survival Commission Role of the SSC The Species Survival Commission (SSC) is IUCN's primary source of the 4. To provide advice, information, and expertise to the Secretariat of the scientific and technical information required for the maintenance of biologi- Convention on International Trade in Endangered Species of Wild Fauna cal diversity through the conservation of endangered and vulnerable species and Flora (CITES) and other international agreements affecting conser- of fauna and flora, whilst recommending and promoting measures for their vation of species or biological diversity. conservation, and for the management of other species of conservation con- cern. Its objective is to mobilize action to prevent the extinction of species, 5. To carry out specific tasks on behalf of the Union, including: sub-species and discrete populations of fauna and flora, thereby not only maintaining biological diversity but improving the status of endangered and • coordination of a programme of activities for the conservation of bio- vulnerable species. logical diversity within the framework of the IUCN Conservation Programme. Objectives of the SSC • promotion of the maintenance of biological diversity by monitoring 1. To participate in the further development, promotion and implementation the status of species and populations of conservation concern. of the World Conservation Strategy; to advise on the development of IUCN's Conservation Programme; to support the implementation of the • development and review of conservation action plans and priorities Programme' and to assist in the development, screening, and monitoring for species and their populations. -

Morphological Facilitators for Vocal Learning Explored Through the Syrinx Anatomy of A
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.11.902734; this version posted January 13, 2020. The copyright holder for this1 preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Morphological facilitators for vocal learning explored through the syrinx anatomy of a 2 basal hummingbird 3 Amanda Monte1, Alexander F. Cerwenka2, Bernhard Ruthensteiner2, Manfred Gahr1, and 4 Daniel N. Düring1,3,4* 5 * Correspondence: [email protected] 6 1 – Department of Behavioural Neurobiology, Max Planck Institute for Ornithology, 7 Seewiesen, Germany 8 2 – SNSB-Bavarian State Collection of Zoology, Munich, Germany 9 3 – Institute of Neuroinformatics, ETH Zurich & University of Zurich, Zurich, Switzerland 10 4 – Neuroscience Center Zurich (ZNZ), Zurich, Switzerland 11 Abstract 12 Vocal learning is a rare evolutionary trait that evolved independently in three avian clades: 13 songbirds, parrots, and hummingbirds. Although the anatomy and mechanisms of sound 14 production in songbirds are well understood, little is known about the hummingbird’s vocal 15 anatomy. We use high-resolution micro-computed tomography (μCT) and microdissection to 16 reveal the three-dimensional structure of the syrinx, the vocal organ of the black jacobin 17 (Florisuga fusca), a phylogenetically basal hummingbird species. We identify three unique 18 features of the black jacobin’s syrinx: (i) a shift in the position of the syrinx to the outside of 19 the thoracic cavity and the related loss of the sterno-tracheal muscle, (ii) complex intrinsic 20 musculature, oriented dorso-ventrally, and (iii) ossicles embedded in the medial vibratory 21 membranes. -
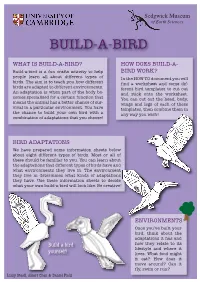
Build-A-Bird
Sedgwick Museum of Earth Sciences BUILD-A-BIRD WHAT IS BUILD-A-BIRD? HOW DOES BUILD-A- Build-a-bird is a fun crafts activity to help BIRD WORK? people learn all about different types of In the HOW TO document you will birds. The aim is to teach you how different find a worksheet and some dif- birds are adapted to different environments. ferent bird templates to cut out An adaptation is when part of the body be- and stick onto the worksheet. comes specialised for a certain function that You can cut out the head, body, means the animal has a better chance of sur- wings and legs of each of these vival in a particular environment. You have templates, then combine them in the chance to build your own bird with a any way you wish! combination of adaptations that you choose! BIRD ADAPTATIONS We have prepared some information sheets below about eight different types of birds. Most or all of these should be familiar to you. You can learn about the adaptations that different types of birds have and what environments they live in. The environment they live in determines what kinds of adaptations they have. Use these information sheets to decide what your own build-a-bird will look like. Be creative! ENVIRONMENTS Once you’ve built your bird, think about the adaptations it has and Build a bird how they relate to its lifestyle and where it yourself! lives. What food might it eat? How does it move around? Can it fly, swim or run? Lizzy Steell, Albert Chen & Daniel Field Sedgwick Museum of Earth Sciences BUILD-A-BIRD: GROUND BIRDS BILL Many ground birds have a gently curved bill (beak) they may use ENVIRONMENT to peck at the ground, eating a Ground birds may spend most diet of plants, seeds and insects. -

Tracheal Disease in Avians: Preparation and Treatment
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Tracheal disease in avians: preparation and treatment Author : Neil Forbes Categories : Exotics, Vets Date : July 20, 2015 The trachea extends from the base of the tongue to the primary bronchi, just within the chest cavity. It runs opposed to, and parallel with, the oesophagus. Birds do not have an epiglottis – instead, the rima glottis functions to open and close, protecting the airway when swallowing and otherwise enabling respiration. The rima glottis is located in the caudal tongue. The proximal trachea attaches to the rima glottis (joining the tongue to the trachea). The avian larynx (unlike in mammals) is not involved in vocalisation – this function is performed by the syrinx (situated at the tracheal bifurcation in the proximal thoracic cavity). Thyroid and epiglottic cartilages are absent. The larynx functions to open the glottis during inspiration, and to close it during swallowing (Figures 1 and 3). Figure 1. African grey parrot glottis – entrance into the trachea. 1 / 11 Figure 2. Close-up of a psittacine glottis. Figure 3. Papilloma lesion on the glottis of a hawk’s head, caused by psittacine herpesvirus. There is significant tracheal variation between species. The level of the bifurcation into the primary bronchi can vary (penguins and spoonbills are more proximal), the length and convolutions of the trachea vary inside or outside the sternum (55 species, mainly cranes and swans, possess complex loops), while some ducks have distended syringeal bulla (an ovoid resonance chamber, created as a dilation from the lower trachea). For voice alteration, such structures are absent in psittacines. -

The Vocal Organ of Hummingbirds Shows Convergence with Songbirds Tobias Riede & Christopher R
www.nature.com/scientificreports OPEN The vocal organ of hummingbirds shows convergence with songbirds Tobias Riede & Christopher R. Olson* How sound is generated in the hummingbird syrinx is largely unknown despite their complex vocal behavior. To fll this gap, syrinx anatomy of four North American hummingbird species were investigated by histological dissection and contrast-enhanced microCT imaging, as well as measurement of vocalizations in a heliox atmosphere. The placement of the hummingbird syrinx is uniquely located in the neck rather than inside the thorax as in other birds, while the internal structure is bipartite with songbird-like anatomical features, including multiple pairs of intrinsic muscles, a robust tympanum and several accessory cartilages. Lateral labia and medial tympaniform membranes consist of an extracellular matrix containing hyaluronic acid, collagen fbers, but few elastic fbers. Their upper vocal tract, including the trachea, is shorter than predicted for their body size. There are between- species diferences in syrinx measurements, despite similar overall morphology. In heliox, fundamental frequency is unchanged while upper-harmonic spectral content decrease in amplitude, indicating that syringeal sounds are produced by airfow-induced labia and membrane vibration. Our fndings predict that hummingbirds have fne control of labia and membrane position in the syrinx; adaptations that set them apart from closely related swifts, yet shows convergence in their vocal organs with those of oscines. Due to their small body size, hummingbirds have experienced selection for a number of traits that have set them apart from other avian lineages1. Various modes of acoustic communication are among those traits2. For example, some hummingbirds use elements of their plumage to generate sounds for efective communication with con- specifcs3.