Systematic Relationships and Biogeography of the Tracheophone Suboscines (Aves: Passeriformes)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Pycnonotidae: Andropadus) Supports a Montane Speciation Model
Recent diversi¢cation in African greenbuls (Pycnonotidae: Andropadus) supports a montane speciation model MICHAEL S. ROY Zoological Institute, University of Copenhagen, Universitetsparken 15, DK 2100, Denmark SUMMARY It is generally accepted that accentuated global climatic cycles since the Plio-Pleistocene (2.8 Ma ago) have caused the intermittent fragmentation of forest regions into isolated refugia thereby providing a mechanism for speciation of tropical forest biota contained within them. However, it has been assumed that this mechanism had its greatest e¡ect in the species rich lowland regions. Contrary evidence from molecular studies of African and South American forest birds suggests that areas of recent intensive speciation, where mostly new lineages are clustered, occur in discrete tropical montane regions, while lowland regions contain mostly old species. Two predictions arise from this ¢nding. First, a species phylogeny of an avian group, represented in both lowland and montane habitats, should be ordered such that montane forms are represented by the most derived characters. Second, montane speciation events should predominate within the past 2.8 Ma. In order to test this model I have investigated the evolutionary history of the recently radiated African greenbuls (genus Andropadus), using a molecular approach. This analysis ¢nds that montane species are a derived monophyletic group when compared to lowland species of the same genus and recent speciation events (within the Plio-Pleistocene) have exclusively occurred in montane regions. These data support the view that montane regions have acted as centres of speciation during recent climatic instability. 1. I NTRODUCTION biodiversity of tropical lowland forests was the `refuge theory' (Ha¡er 1969, 1974; Diamond & Hamilton The most signi¢cant geological changes that a¡ected 1980; Mayr & O'Hara 1986; Crowe & Crowe 1982; re- the biota of Africa during the Tertiary were: (i) a views in Prance 1982; Whitmore & Prance 1987). -

The Birds of Reserva Ecológica Guapiaçu (REGUA)
Cotinga 33 The birds of Reserva Ecológica Guapiaçu (REGUA), Rio de Janeiro, Brazil Leonardo Pimentel and Fábio Olmos Received 30 September 2009; final revision accepted 15 December 2010 Cotinga 33 (2011): OL 8–24 published online 16 March 2011 É apresentada uma lista da avifauna da Reserva Ecológica de Guapiaçu (REGUA), uma reserva privada de 6.500 ha localizada no município de Cachoeiras de Macacu, vizinha ao Parque Estadual dos Três Picos, Estação Ecológica do Paraíso e Parque Nacional da Serra dos Órgãos, parte de um dos maiores conjuntos protegidos do Estado do Rio de Janeiro. Foram registradas um total de 450 espécies de aves, das quais 63 consideradas de interesse para conservação, como Leucopternis lacernulatus, Harpyhaliaetus coronatus, Triclaria malachitacea, Myrmotherula minor, Dacnis nigripes, Sporophila frontalis e S. falcirostris. A reserva também está desenvolvendo um projeto de reintrodução dos localmente extintos Crax blumembachii e Aburria jacutinga, e de reforço das populações locais de Tinamus solitarius. The Atlantic Forest of eastern Brazil and Some information has been published on neighbouring Argentina and Paraguay is among the birds of lower (90–500 m) elevations in the the most imperilled biomes in the world. At region10,13, but few areas have been subject to least 188 bird species are endemic to it, and 70 long-term surveys. Here we present the cumulative globally threatened birds occur there, most of them list of a privately protected area, Reserva Ecológica endemics4,8. The Atlantic Forest is not homogeneous Guapiaçu (REGUA), which includes both low-lying and both latitudinal and longitudinal gradients parts of the Serra dos Órgãos massif and nearby account for diverse associations of discrete habitats higher ground, now mostly incorporated within and associated bird communities. -

Acari: Syringophilidae: Picobiinae) Associated with Woodcreeper Birds (Passeriformes: Dendrocolaptidae)
Zootaxa 3406: 59–66 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) New picobiin mites (Acari: Syringophilidae: Picobiinae) associated with woodcreeper birds (Passeriformes: Dendrocolaptidae) MACIEJ SKORACKI1, 3& PIOTR SOLARCZYK2 1Department of Animal Morphology, Faculty of Biology, Adam Mickiewicz University, Umultowska 89, 61–614 Poznan, Poland 2Department of Biology and Medical Parasitology, Poznan University of Medical Sciences, Faculty of Medicine I, 10 Fredry Street, Poznan, Poland 3Corresponding author. E-mail: [email protected] Abstract Two new species belonging to the genus Rafapicobia Skoracki, 2011 (Syringophilidae: Picobiinae) collected from birds of the family Dendrocolaptidae are described: 1) Rafapicobia dendrocolaptesi sp. nov. from Dendrocolaptes platyrostris Spix (type host) in Paraguay and from D. picumnus Lichtenstein in Argentina; 2) Rafapicobia lepidocolaptesi sp. nov. from Lepidocolaptes affinis (Lafresnaye) (type host) in Ecuador and Venezuela and from L. souleyetii (Des Murs) in Co- lombia. Syringophilid mites are recorded from woodcreepers for the first time. Key words: Acari, Syringophilidae, Picobiinae, quill mites, ectoparasites, birds, Dendrocolaptidae Introduction The subfamily Picobiinae Johnson and Kethley (Acariformes: Cheyletoidea: Syringophilidae) is potentially taxo- nomically diverse group of the syringophilid mites parasitising birds. All members of this subfamily occupy exclu- sively quills of body -

Aves: Rhinocryptidae): in and out Per G
Circumscription of a monophyletic family for the tapaculos (Aves: Rhinocryptidae): in and out Per G. P. Ericson, Storrs L. Olson, Martin Irestedt, Herculano Alvarenga, Jon Fjeldså To cite this version: Per G. P. Ericson, Storrs L. Olson, Martin Irestedt, Herculano Alvarenga, Jon Fjeldså. Circumscrip- tion of a monophyletic family for the tapaculos (Aves: Rhinocryptidae): in and out. Journal für Ornithologie = Journal of Ornithology, Springer Verlag, 2009, 151 (2), pp.337-345. 10.1007/s10336- 009-0460-9. hal-00568355 HAL Id: hal-00568355 https://hal.archives-ouvertes.fr/hal-00568355 Submitted on 23 Feb 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 2 3 4 Circumscription of a monophyletic family for the tapaculos 5 (Aves: Rhinocryptidae): Psiloramphus in and Melanopareia out 6 7 8 Per G. P. Ericson 1, 6, Storrs L. Olson 2, Martin Irestedt 3, Herculano Alvarenga 4, 9 and Jon Fjeldså 5 10 11 12 13 14 15 1 Department of Vertebrate Zoology, Swedish Museum of Natural History, P.O. Box 16 50007, SE–10405 Stockholm, Sweden 17 18 2 Division of Birds, Department of Vertebrate Zoology, National Museum of Natural 19 History, P.O. -

BIOLOGICAL INVENTORIES REPORTS ARE PUBLISHED BY: Betty Moore Foundation./This Publication Has Been Funded in Part by the Gordon and Betty Moore Foundation
biological rapid inventories 12 Perú: Ampiyacu, Apayacu, Yaguas, Medio Putumayo Nigel Pitman, Richard Chase Smith, Corine Vriesendorp, Debra Moskovits, Renzo Piana, Guillermo Knell y/and Tyana Wachter, editores/editors ABRIL/APRIL 2004 Instituciones y Comunidades Participantes/ Participating Institutions and Communities The Field Museum Comunidades Nativas de los ríos Ampiyacu, Apayacu y Medio Putumayo/Indigenous Communities of the Ampiyacu, Apayacu and Medio Putumayo rivers FECONA FECONAFROPU Instituto del Bien Común Servicio Holandés de Cooperación al Desarrollo/ SNV Netherlands Development Organization Centro de Conservación, Investigación y Manejo de Áreas Naturales (CIMA-Cordillera Azul) Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos LOS INVENTARIOS BIOLÓGICOS RÁPIDOS SON PUBLICADOS POR / Esta publicación ha sido financiada en parte por Gordon and RAPID BIOLOGICAL INVENTORIES REPORTS ARE PUBLISHED BY: Betty Moore Foundation./This publication has been funded in part by the Gordon and Betty Moore Foundation. THE FIELD MUSEUM Environmental and Conservation Programs Cita Sugerida/Suggested Citation: Pitman, N., R. C. Smith, 1400 South Lake Shore Drive C. Vriesendorp, D. Moskovits, R. Piana, G. Knell & T. Wachter Chicago, Illinois 60605-2496 USA (eds.). 2004. Perú: Ampiyacu, Apayacu, Yaguas, Medio Putumayo. T 312.665.7430, F 312.665.7433 Rapid Biological Inventories Report 12. Chicago, Illinois: www.fieldmuseum.org The Field Museum. Créditos Fotográficos/Photography credits: Editores/Editors: Nigel Pitman, Richard Chase Smith, Corine Vriesendorp, Debra Moskovits, Renzo Piana, Carátula/Cover: Un padre Bora con sus hijos atienden un taller en Guillermo Knell, Tyana Wachter Boras de Brillo Nuevo. Foto de Alvaro del Campo./A Bora father and his children attend a workshop in Boras de Brillo Nuevo. -

Bird Responses to Shade Coffee Production
Animal Conservation (2004) 7, 169–179 C 2004 The Zoological Society of London. Printed in the United Kingdom DOI:10.1017/S1367943004001258 Bird responses to shade coffee production Cesar´ Tejeda-Cruz and William J. Sutherland Centre for Ecology, Evolution and Conservation, School of Biological Sciences, University of East Anglia, Norwich NR4 7TJ, UK (Received 2 June 2003; accepted 16 October 2003) Abstract It has been documented that certain types of shade coffee plantations have both biodiversity levels similar to natural forest and high concentrations of wintering migratory bird species. These findings have triggered a campaign to promote shade coffee as a means of protecting Neotropical migratory birds. Bird censuses conducted in the El Triunfo Biosphere reserve in southern Mexico have confirmed that shade coffee plantations may have bird diversity levels similar to, or higher than, natural forest. However, coffee and forest differed in species composition. Species with a high sensitivity to disturbance were significantly more diverse and abundant in primary ecosystems. Neotropical migratory birds, granivorous and omnivorous species were more abundant in disturbed habitats. Insectivorous bird species were less abundant only in shaded monoculture. Foraging generalists and species that prefer the upper foraging stratum were more abundant in disturbed habitats, while a decline in low and middle strata foragers was found there. Findings suggests that shade coffee may be beneficial for generalist species (including several migratory species), but poor for forest specialists. Although shade coffee plantations may play an important role in maintaining local biodiversity, and as buffer areas for forest patches, promotion of shade coffee may lead to the transformation of forest into shade coffee, with the consequent loss of forest species. -

Abstract Book
Welcome to the Ornithological Congress of the Americas! Puerto Iguazú, Misiones, Argentina, from 8–11 August, 2017 Puerto Iguazú is located in the heart of the interior Atlantic Forest and is the portal to the Iguazú Falls, one of the world’s Seven Natural Wonders and a UNESCO World Heritage Site. The area surrounding Puerto Iguazú, the province of Misiones and neighboring regions of Paraguay and Brazil offers many scenic attractions and natural areas such as Iguazú National Park, and provides unique opportunities for birdwatching. Over 500 species have been recorded, including many Atlantic Forest endemics like the Blue Manakin (Chiroxiphia caudata), the emblem of our congress. This is the first meeting collaboratively organized by the Association of Field Ornithologists, Sociedade Brasileira de Ornitologia and Aves Argentinas, and promises to be an outstanding professional experience for both students and researchers. The congress will feature workshops, symposia, over 400 scientific presentations, 7 internationally renowned plenary speakers, and a celebration of 100 years of Aves Argentinas! Enjoy the book of abstracts! ORGANIZING COMMITTEE CHAIR: Valentina Ferretti, Instituto de Ecología, Genética y Evolución de Buenos Aires (IEGEBA- CONICET) and Association of Field Ornithologists (AFO) Andrés Bosso, Administración de Parques Nacionales (Ministerio de Ambiente y Desarrollo Sustentable) Reed Bowman, Archbold Biological Station and Association of Field Ornithologists (AFO) Gustavo Sebastián Cabanne, División Ornitología, Museo Argentino -

Distribution of Birds Along an Elevational Gradient in the Atlantic Forest of Brazil: Implications for the Conservation of Endemic and Endangered Species
Bird Conservation International (1999) 9:235-253. © BirdLife International 1999 Distribution of birds along an elevational gradient in the Atlantic forest of Brazil: implications for the conservation of endemic and endangered species JAQUELINE M. GOERCK Summary In this study I compare bird communities along an elevational gradient in an Atlantic forest remnant (Pico do Corcovado in Ubatuba) in coastal Sao Paulo state, Brazil. Forests at low elevations are structurally more complex and more diverse in plant species than those along the slopes and at higher elevations in this remnant. Consequently it is hypothesized that low elevation forests contain a greater diversity of bird species. Results from the study in the Corcovado area show clear differences in the distribution of forest birds along the elevational gradient from both qualitative and quantitative aspects. The structurally more complex forest at low elevations contains the most diverse avifauna, including several of the rarest and most threatened species. The importance of this remnant as a whole is apparent due to the high diversity observed (254 species), the high proportion of endemic species, and the extent to which the avifauna is endangered. Protection of forests at all elevations along the Serra do Mar is required to maintain diversity of bird species, particularly the many endemic and endangered species restricted to specific elevations. Resumo Comunidades de aves foram comparadas ao longo de um gradiente altitudinal em um remanescente florestal de Mata Atlantica (Pico do Corcovado em Ubatuba) na costa do estado de Sao Paulo. As florestas de baixada sao mais diversas e mais complexas estruturalmente do que as florestas de encosta ou de altitude e consequentemente espera-se que as florestas de baixada possuam uma maior diversidade de aves. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -
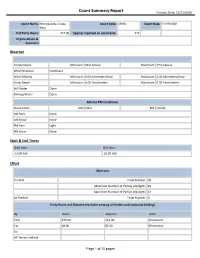
Count Summary Report Printout Date: 11/11/2016
Count Summary Report Printout Date: 11/11/2016 Count Name: Monteverde, Costa Count Code: CRMO Count Date: 12/19/2001 Rica # of Party Hours: 257.00 Species reported on count date: 376 Organizations & Sponsors: Weather Temperature Minimum: 59.0 Celsius Maximum: 77.0 Celsius Wind Direction Northeast Wind Velocity Minimum: 0.00 Kilometers/hour Maximum: 6.00 Kilometers/hour Snow Depth Minimum: 0.00 Centimeters Maximum: 0.00 Centimeters Still Water Open Moving Water Open AM and PM Conditions Cloud Cover AM: Clear PM: Cloudy AM Rain None AM Snow None PM Rain Light PM Snow None Start & End Times Start time End time 12:00 AM 10:00 AM Effort Observers In Field Total Number: 70 Minimum Number of Parties (daylight): 19 Maximum Number of Parties (daylight): 24 At Feeders Total Number: 0 Party Hours and Distance (excludes viewing at feeders and nocturnal birding) By Hours Distance Units Foot 239.00 114.00 Kilometers Car 18.00 85.00 Kilometers Air All-Terrain Vehicle Page 1 of 12 pages Count Summary Report Printout Date: 11/11/2016 Bicycle Dog Sled Golfcart Horseback Motorized Boat Non-Motorized Boat Skis/Xc-Skis Snowmachine Snowshoe Wheelchair Other Time and Distance Hours Distance Units At Feeders 0.00 Nocturnal Birding 17.00 26.00 Miles Total Party 257.00 199.00 Kilometers Checklist Species Number Number/Party Hrs. Flags Editorial Codes Highland Tinamou 3 0.0117 Great Tinamou 2 0.0078 Gray-headed Chachalaca 33 0.1284 Crested Guan 20 0.0778 Black Guan 18 0.0700 Black-breasted Wood-Quail 56 0.2179 Least Grebe 5 0.0195 Anhinga 1 0.0039 Fasciated Tiger-Heron -

Table of Contents
FRAGMENTATION SENSITIVITY AND ITS CONSEQUENCES ON DEMOGRAPHY AND HOST–ECTOPARASITE DYNAMICS IN AMAZONIAN BIRDS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The School of Renewable Natural Resources by Erik I. Johnson B.S., Dickinson College, 2001 M.S., Louisiana State University, 2006 May 2011 DEDICATION I dedicate this manuscript to my wife, my partner in life, and my closest friend, Ceci Johnson, who encourages me to follow my dreams and gives me the inspiration to push forward in good and hard times. I will always appreciate and never forget her unending patience and love through this journey. ii ACKNOWLEDGEMENTS First and foremost, I thank my advisor and Amazon guru, Dr. Phil Stouffer. His endless insight and patience is remarkable and I am so grateful to him for including me in his lab. My fellow labmates, past and present, have always been there for me and I appreciate all of their council and friendship. These fine people that I have had a privalenge to work with include Matt Brooks, David Brown, Emma DeLeon, Jenny DiMiceli, Lynn Duda, Dave Fox, Karl Mokross, Falyn Owens, Laura Palasz, Luke Powell, Jared Wolfe, and Jason Zoller. Luke, Karl, and Jared: it has been incredible getting to spend time with you in the Amazon – best of luck with your continued work there. I cannot wait to see the cool things you discover. I am very thankful to my committee, Dr. Van Remsen, Dr. -

Lepidocolaptes Angustirostris) in a Southern Temperate Forest of Central-East Argentina
Studies on Neotropical Fauna and Environment ISSN: 0165-0521 (Print) 1744-5140 (Online) Journal homepage: https://www.tandfonline.com/loi/nnfe20 Nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptes angustirostris) in a southern temperate forest of central-east Argentina Adrián Jauregui, Exequiel Gonzalez & Luciano N. Segura To cite this article: Adrián Jauregui, Exequiel Gonzalez & Luciano N. Segura (2019): Nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptesangustirostris) in a southern temperate forest of central-east Argentina, Studies on Neotropical Fauna and Environment To link to this article: https://doi.org/10.1080/01650521.2019.1590968 Published online: 25 Mar 2019. Submit your article to this journal View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=nnfe20 STUDIES ON NEOTROPICAL FAUNA AND ENVIRONMENT https://doi.org/10.1080/01650521.2019.1590968 ORIGINAL ARTICLE Nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptes angustirostris) in a southern temperate forest of central-east Argentina Adrián Jauregui, Exequiel Gonzalez and Luciano N. Segura Sección Ornitología, Museo de La Plata, Universidad Nacional de La Plata-CONICET, La Plata, Buenos Aires, Argentina ABSTRACT ARTICLE HISTORY We present data on the nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptes Received 24 September 2018 angustirostris) in a natural forest in central-east Argentina. A total of 18 nests were found during Accepted 27 February 2019 – four breeding seasons (2015 2019; from September to January), located in cavities (natural, KEYWORDS artificial and woodpecker cavities). The incubation period lasted 16 days and eggs were larger Breeding parameters; than those from northern populations.