Caractérisation De Tgzfp2, Une Nouvelle Protéine À
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Life: the Science of Biology
Lecture Notebook to accompany SINAUER ASSOCIATES, INC. MACMILLAN Copyright © 2014 Sinauer Associates, Inc. Cover photograph © Alex Mustard/naturepl.com. This document may not be modified or distributed (either electronically or on paper) without the permission of the publisher, with the following exception: Individual users may enter their own notes into this document and may print it for their own personal use. The Origin and Diversification of 0027 Eukaryotes 27.1 A Hypothetical Sequence for the Evolution of the Eukaryotic Cell (Page 550) 1 The protective Cell wall cell wall was lost. DNA 2 Infolding of the plasma membrane added surface area without increasing the cell’s volume. 3 Cytoskeleton (microfilament and microtubules) formed. 4 Internal membranes studded with ribosomes formed. 5 As regions of the infolded plasma membrane enclosed the cell’s DNA, a precursor of a nucleus formed. 6 Microtubules from the cytoskeleton formed the eukaryotic flagellum, 7 Early digestive vacuoles enabling propulsion. evolved into lysosomes using enzymes from the early endoplasmic reticulum. 8 Mitochondria formed through endosymbiosis with a proteobacterium. 9 Endosymbiosis with cyanobacteria led to the development of chloroplasts. Flagellum To add your own notes to any page, use Adobe Reader’s Chloroplast Typewriter feature, accessible via the Typewriter bar at Mitochondrion the top of the window. (Requires Adobe Reader 8 or later. Adobe Reader can be downloaded free of charge from the Nucleus Adobe website: http://get.adobe.com/reader.) LIFE2 The Science of Biology 10E Sadava © 2014 Sinauer Associates, Inc. Sinauer Associates Morales Studio Figure 27.01 05-24-12 Chapter 27 | The Origin and Diversification of Eukaryotes 3 (A) Primary endosymbiosis Eukaryote Cyanobacterium Cyanobacterium outer membrane Peptidoglycan Cyanobacterium inner membrane Host cell nucleus Chloroplast Peptidoglycan has been lost except in glaucophytes. -

Gaits in Paramecium Escape
Transitions between three swimming gaits in Paramecium escape Amandine Hamela, Cathy Fischb, Laurent Combettesc,d, Pascale Dupuis-Williamsb,e, and Charles N. Barouda,1 aLadHyX and Department of Mechanics, Ecole Polytechnique, Centre National de la Recherche Scientifique, 91128 Palaiseau cedex, France; bActions Thématiques Incitatives de Genopole® Centriole and Associated Pathologies, Institut National de la Santé et de la Recherche Médicale Unité-Université d’Evry-Val-d’Essonne Unité U829, Université Evry-Val d'Essonne, Bâtiment Maupertuis, Rue du Père André Jarlan, 91025 Evry, France; cInstitut National de la Santé et de la Recherche Médicale Unité UMRS-757, Bâtiment 443, 91405 Orsay, France; dSignalisation Calcique et Interactions Cellulaires dans le Foie, Université de Paris-Sud, Bâtiment 443, 91405 Orsay, France; and eEcole Supérieure de Physique et de Chimie Industrielles ParisTech, 10 rue Vauquelin, 75005 Paris, France Edited* by Harry L. Swinney, University of Texas at Austin, Austin, TX, and approved March 8, 2011 (received for review November 10, 2010) Paramecium and other protists are able to swim at velocities reach- or in the switching between the different swimming behaviors ing several times their body size per second by beating their cilia (11, 13–17). in an organized fashion. The cilia beat in an asymmetric stroke, Below we show that Paramecium may also use an alternative to which breaks the time reversal symmetry of small scale flows. Here cilia to propel itself away from danger, which is based on tricho- we show that Paramecium uses three different swimming gaits to cyst extrusion. Trichocysts are exocytotic organelles, which are escape from an aggression, applied in the form of a focused laser regularly distributed along the plasma membrane in Paramecium heating. -

Tuberlatum Coatsi Gen. N., Sp. N. (Alveolata, Perkinsozoa), a New
Protist, Vol. 170, 82–103, February 2019 http://www.elsevier.de/protis Published online date 21 December 2018 ORIGINAL PAPER Tuberlatum coatsi gen. n., sp. n. (Alveolata, Perkinsozoa), a New Parasitoid with Short Germ Tubes Infecting Marine Dinoflagellates 1 Boo Seong Jeon, and Myung Gil Park LOHABE, Department of Oceanography, Chonnam National University, Gwangju 61186, Republic of Korea Submitted October 16, 2018; Accepted December 15, 2018 Monitoring Editor: Laure Guillou Perkinsozoa is an exclusively parasitic group within the alveolates and infections have been reported from various organisms, including marine shellfish, marine dinoflagellates, freshwater cryptophytes, and tadpoles. Despite its high abundance and great genetic diversity revealed by recent environmental rDNA sequencing studies, Perkinsozoa biodiversity remains poorly understood. During the intensive samplings in Korean coastal waters during June 2017, a new parasitoid of dinoflagellates was detected and was successfully established in culture. The new parasitoid was most characterized by the pres- ence of two to four dome-shaped, short germ tubes in the sporangium. The opened germ tubes were biconvex lens-shaped in the top view and were characterized by numerous wrinkles around their open- ings. Phylogenetic analyses based on the concatenated SSU and LSU rDNA sequences revealed that the new parasitoid was included in the family Parviluciferaceae, in which all members were comprised of two separate clades, one containing Parvilucifera species (P. infectans, P. corolla, and P. rostrata), and the other containing Dinovorax pyriformis, Snorkelia spp., and the new parasitoid from this study. Based on morphological, ultrastructural, and molecular data, we propose to erect a new genus and species, Tuberlatum coatsi gen. -
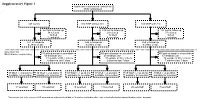
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Why the –Omic Future of Apicomplexa Should Include Gregarines Julie Boisard, Isabelle Florent
Why the –omic future of Apicomplexa should include Gregarines Julie Boisard, Isabelle Florent To cite this version: Julie Boisard, Isabelle Florent. Why the –omic future of Apicomplexa should include Gregarines. Biology of the Cell, Wiley, 2020, 10.1111/boc.202000006. hal-02553206 HAL Id: hal-02553206 https://hal.archives-ouvertes.fr/hal-02553206 Submitted on 24 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Article title: Why the –omic future of Apicomplexa should include Gregarines. Names of authors: Julie BOISARD1,2 and Isabelle FLORENT1 Authors affiliations: 1. Molécules de Communication et Adaptation des Microorganismes (MCAM, UMR 7245), Département Adaptations du Vivant (AVIV), Muséum National d’Histoire Naturelle, CNRS, CP52, 57 rue Cuvier 75231 Paris Cedex 05, France. 2. Structure et instabilité des génomes (STRING UMR 7196 CNRS / INSERM U1154), Département Adaptations du vivant (AVIV), Muséum National d'Histoire Naturelle, CP 26, 57 rue Cuvier 75231 Paris Cedex 05, France. Short Title: Gregarines –omics for Apicomplexa studies -

Tracing the Origin of Planktonic Protists in an Ancient Lake
microorganisms Article Tracing the Origin of Planktonic Protists in an Ancient Lake Nataliia V. Annenkova 1,* , Caterina R. Giner 2,3 and Ramiro Logares 2,* 1 Limnological Institute Siberian Branch of the Russian Academy of Sciences 3, Ulan-Batorskaya St., 664033 Irkutsk, Russia 2 Institute of Marine Sciences (ICM), CSIC, Passeig Marítim de la Barceloneta, 37-49, ES08003 Barcelona, Spain; [email protected] 3 Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver, BC V6T 1Z4, Canada * Correspondence: [email protected] (N.V.A.); [email protected] (R.L.) Received: 26 February 2020; Accepted: 7 April 2020; Published: 9 April 2020 Abstract: Ancient lakes are among the most interesting models for evolution studies because their biodiversity is the result of a complex combination of migration and speciation. Here, we investigate the origin of single celled planktonic eukaryotes from the oldest lake in the world—Lake Baikal (Russia). By using 18S rDNA metabarcoding, we recovered 1414 Operational Taxonomic Units (OTUs) belonging to protists populating surface waters (1–50 m) and representing pico/nano-sized cells. The recovered communities resembled other lacustrine freshwater assemblages found elsewhere, especially the taxonomically unclassified protists. However, our results suggest that a fraction of Baikal protists could belong to glacial relicts and have close relationships with marine/brackish species. Moreover, our results suggest that rapid radiation may have occurred among some protist taxa, partially mirroring what was already shown for multicellular organisms in Lake Baikal. We found 16% of the OTUs belonging to potential species flocks in Stramenopiles, Alveolata, Opisthokonta, Archaeplastida, Rhizaria, and Hacrobia. -

Lateral Gene Transfer of Anion-Conducting Channelrhodopsins Between Green Algae and Giant Viruses
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.15.042127; this version posted April 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 5 Lateral gene transfer of anion-conducting channelrhodopsins between green algae and giant viruses Andrey Rozenberg 1,5, Johannes Oppermann 2,5, Jonas Wietek 2,3, Rodrigo Gaston Fernandez Lahore 2, Ruth-Anne Sandaa 4, Gunnar Bratbak 4, Peter Hegemann 2,6, and Oded 10 Béjà 1,6 1Faculty of Biology, Technion - Israel Institute of Technology, Haifa 32000, Israel. 2Institute for Biology, Experimental Biophysics, Humboldt-Universität zu Berlin, Invalidenstraße 42, Berlin 10115, Germany. 3Present address: Department of Neurobiology, Weizmann 15 Institute of Science, Rehovot 7610001, Israel. 4Department of Biological Sciences, University of Bergen, N-5020 Bergen, Norway. 5These authors contributed equally: Andrey Rozenberg, Johannes Oppermann. 6These authors jointly supervised this work: Peter Hegemann, Oded Béjà. e-mail: [email protected] ; [email protected] 20 ABSTRACT Channelrhodopsins (ChRs) are algal light-gated ion channels widely used as optogenetic tools for manipulating neuronal activity 1,2. Four ChR families are currently known. Green algal 3–5 and cryptophyte 6 cation-conducting ChRs (CCRs), cryptophyte anion-conducting ChRs (ACRs) 7, and the MerMAID ChRs 8. Here we 25 report the discovery of a new family of phylogenetically distinct ChRs encoded by marine giant viruses and acquired from their unicellular green algal prasinophyte hosts. -

Assessing the Efficiency of Molecular Markers for the Species Identification of Gregarines Isolated from the Mealworm and Super Worm Midgut
Microorganisms 2018, 6, 119 1 of 20 Article Assessing the Efficiency of Molecular Markers for the Species Identification of Gregarines Isolated from the Mealworm and Super Worm Midgut Chiara Nocciolini 1, Claudio Cucini 2, Chiara Leo 2, Valeria Francardi 3, Elena Dreassi 4 and Antonio Carapelli 2,* 1 Polo d’Innovazione di Genomica Genetica e Biologia, 53100 Siena, Italy; [email protected] 2 Department of Life Sciences, University of Siena, 53100 Siena, Italy; [email protected] (C.C.); [email protected] (C.L.) 3 Consiglio per la ricerca in agricoltura e analisi dell’economia agraria – Centro di Difesa e Certificazione CREA-DC) Research Centre for Plant Protection and Certification, via di Lanciola 12/A, 50125 Firenze, Italy; [email protected] 4 Department of Biotechnology, Chemistry and Pharmacy, University of Siena, 53100 Siena, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0577-234-410 Received: 28 September 2018; Accepted: 23 November 2018; Published: 27 November 2018 Abstract: Protozoa, of the taxon Gregarinasina, are a heterogeneous group of Apicomplexa that includes ~1600 species. They are parasites of a large variety of both marine and terrestrial invertebrates, mainly annelids, arthropods and mollusks. Unlike coccidians and heamosporidians, gregarines have not proven to have a negative effect on human welfare; thus, they have been poorly investigated. This study focuses on the molecular identification and phylogeny of the gregarine species found in the midgut of two insect species that are considered as an alternative source of animal proteins for the human diet: the mealworm Tenebrio molitor, and the super-worm Zophobas atratus (Coleoptera: Tenebrionidae). -

Chloroplast Structure of the Cryptophyceae
CHLOROPLAST STRUCTURE OF THE CRYPTOPHYCEAE Evidence for Phycobiliproteins within Intrathylakoidal Spaces E . GANTT, M . R . EDWARDS, and L . PROVASOLI From the Radiation Biology Laboratory, Smithsonian Institution, Rockville, Maryland 20852, the Division of Laboratories and Research, New York State Department of Health, Albany, New York 12201, and the Haskins Laboratories, New Haven, Connecticut 06520 ABSTRACT Selective extraction and morphological evidence indicate that the phycobiliproteins in three Cryptophyceaen algae (Chroomonas, Rhodomonas, and Cryptomonas) are contained within intrathylakoidal spaces and are not on the stromal side of the lamellae as in the red and blue-green algae . Furthermore, no discrete phycobilisome-type aggregates have thus far been observed in the Cryptophyceae . Structurally, although not necessarily functionally, this is a radical difference . The width of the intrathylakoidal spaces can vary but is gen- erally about 200-300 A . While the thylakoid membranes are usually closely aligned, grana- type fusions do not occur. In Chroomonas these membranes evidence an extensive periodic display with a spacing on the order of 140-160 A . This periodicity is restricted to the mem- branes and has not been observed in the electron-opaque intrathylakoidal matrix . INTRODUCTION The varied characteristics of the cryptomonads (3), Greenwood in Kirk and Tilney-Bassett (10), are responsible for their indefinite taxonomic and Lucas (13) . The thylakoids have a tendency position (17), but at the same time they enhance to be arranged in pairs, that is, for two of them to their status in evolutionary schemes (1, 4) . Their be closely associated with a 30-50 A space between chloroplast structure is distinct from that of every them . -

Gregarines (Apicomplexa: Gregarinasina) Are Monoxenous Parasites of Invertebrates
Gregarines (Apicomplexa: Gregarinasina) are monoxenous parasites of invertebrates. Those found in sand flies (Diptera: Psychodidae) and mosquitoes (Diptera: Culicidae) used to be considered a single eugregarine genus Ascogregarina. Our phylogenetic analyses of the gregarine SSU rDNA, including newly obtained sequences of three species from sand flies, showed that mosquito and sand fly gregarines are closely related to neogregarines, and most importantly, they form two disparate monophyletic groups. Based on these molecular features, accompanied by biological differences, we established a new genus Psychodiella for the gregarines from sand flies, reserving the genus Ascogregarina for the mosquito gregarines. In the new genus, two new species Psychodiella sergenti from Phlebotomus sergenti and Psychodiella tobbi from Phlebotomus tobbi were described. They differ in the life cycles (sexual development of Ps. sergenti is triggered by a blood meal intake) and morphology of their life stages, mainly oocysts. The susceptibility of five sand fly species to both gregarines showed their strict host specificity, as they were able to fully develop and complete the life cycle only in their natural hosts. The life cycle of Ps. sergenti was studied in detail using various microscopical methods. Oocysts are attached to the chorion of sand fly eggs. Sporozoites, with a three- layered pellicle and mucron, attach to the 1st instar larval intestine but are never located intracellularly. In the 4th instar larvae, the gregarines occur in the ectoperitrophic space and later in the intestinal lumen. In adults, the parasites appear in the body cavity, and the sexual development of Ps. sergenti takes place only in blood-fed females; gametocysts attach to the accessory glands and oocysts are injected into their lumen. -

Dinophyceae), with Notes on Feeding Behavior and the Description of the Flagellar Base Area of a Planozygote1
J. Phycol. 42, 434–452 (2006) r 2006 Phycological Society of America DOI: 10.1111/j.1529-8817.2006.00195.x ULTRASTRUCTURE AND LSU rDNA-BASED PHYLOGENY OF ESOPTRODINIUM GEMMA (DINOPHYCEAE), WITH NOTES ON FEEDING BEHAVIOR AND THE DESCRIPTION OF THE FLAGELLAR BASE AREA OF A PLANOZYGOTE1 Anto´nio J. Calado,2 Sandra C. Craveiro Departamento de Biologia, Universidade de Aveiro, P-3810-193 Aveiro, Portugal Niels Daugbjerg and Øjvind Moestrup Department of Phycology, Biological Institute, University of Copenhagen, Øster Farimagsgade 2D, DK-1353 Copenhagen K, Denmark A small, freshwater dinoflagellate with an incom- Markov chains; MP, maximum parsimony; NJ, plete cingulum, identified as Esoptrodinium gemma neighbor-joining; PP, posterior probabilities; PSC, Javornicky´ (5Bernardinium bernardinense sensu au- peduncular striated collar; SRC, striated root con- ctt. non sensu Chodat), was maintained in mixed nective (TSR to LMR); TB, transverse basal body; culture and examined using light and serial section TMR, transverse microtubular root; TMRE, TMR TEM. Vegetative flagellate cells, large cells with two extension; TSR, transverse striated root; TSRM, longitudinal flagella (planozygotes), and cysts were transverse striated root microtubule; VR, ventral examined. The cells displayed a red eyespot near ridge the base of the longitudinal flagellum, made of two or three layers of pigment globules not bounded by a membrane. Yellow-green, band-shaped chloro- plasts, bounded by three membranes and contain- The organism used in this study is a naked dino- ing lamella with three thylakoids, were present in flagellate that would be readily identified as Bern- both flagellate cells and cysts. Most cells had food ardinium bernardinense Chodat by recent dinoflagellate vacuoles, containing phagotrophically ingested floras (Starmach 1974, Matvienko and Litvinenko chlamydomonads or chlorelloid green algae; inges- 1977, Popovsky´ and Pfiester 1990). -

Download File
Acta Protozool. (2012) 51: 305–318 http://www.eko.uj.edu.pl/ap ACTA doi:10.4467/16890027AP.12.024.0784 PROTOZOOLOGICA Morphological Description of Telaepolella tubasferens n. g. n. sp., Isolate ATCC© 50593™, a Filose Amoeba in the Gracilipodida, Amoebozoa Daniel J. G. LAHR1,2*, Gabriela M. KUBIK1*, Anastasia L. GANT1, Jessica GRANT1, O. Roger ANDERSON3 and Laura A. KATZ1,2 1Department of Biological Sciences, Smith College, Northampton, MA, USA; 2Program in Organismic and Evolutionary Biology, University of Massachusetts, Amherst, MA, USA; 3Biology, Lamont-Doherty Earth Observatory of Columbia University, Palisades, New York; * D. J. G. Lahr and G. M. Kubik contributed equally to this work Abstract. We describe the amoeboid isolate ATCC© 50593™ as a new taxon, Telaepolella tubasferens n. g. n. sp. This multinucleated amoeba has filose pseudopods and is superficially similar to members of the vampyrellids (Rhizaria) such as Arachnula impatiens Cien- kowski, 1876, which was the original identification upon deposition. However, previous multigene analyses place this taxon within the Gracilipodida Lahr and Katz 2011 in the Amoebozoa. Here, we document the morphology of this organism at multiple life history stages and provide data underlying the description as a new taxon. We demonstrate that T. tubasferens is distinct from Arachnula and other rhizari- ans (Theratromyxa, Leptophrys) in a suite of morphological characters such as general body shape, relative size of pseudopods, distinction of ecto- and endoplasmic regions, and visibility of nuclei in non-stained cells (an important diagnostic character). Although Amoebozoa taxa generally have lobose pseudopods, genera in Gracilipodida such as Flamella and Filamoeba as well as several organisms previously classified as protosteloid amoebae (e.g.