Magnetic Resonance Force Microscopy for Condensed Matter Issue Date: 2017-07-05 1 Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

J. Robert Schrieffer Strange Quantum Numbers in Condensed Matter
Wednesday, May 1, 2002 3:00 pm APS Auditorium, Building 402, Argonne National Laboratory APS Colloquium home J. Robert Schrieffer Nobel Laureate National High Magnetic Field Laboratory Florida State University, Tallahassee [email protected] http://www.physics.fsu.edu/research/NHMFL.htm Strange Quantum Numbers in Condensed Matter Physics The origin of peculiar quantum numbers in condensed matter physics will be reviewed. The source of spin-charge separation and fractional charge in conducting polymers has to do with solitons in broken symmetry states. For superconductors with an energy gap, which is odd under time reversal, reverse spin-orbital angular momentum pairing occurs. In the fractional quantum Hall effect, quasi particles of fractional charge occur. In superfluid helium 3, a one-way branch of excitations exists if a domain wall occurs in the system. Many of these phenomena occur due to vacuum flow of particles without crossing the excitation of the energy gap. John Robert Schrieffer received his bachelor's degree from Massachusetts Institute of Technology in 1953 and his Ph.D. from the University of Illinois in 1957. In addition, he holds honorary Doctor of Science degrees from universities in Germany, Switzerland, and Israel, and from the University of Pennsylvania, the University of Cincinnati, and the University of Alabama. Since 1992, Dr. Schrieffer has been a professor of Physics at Florida State University and the University of Florida and the Chief Scientist of the National High Magnetic Field Laboratory. He also holds the FSU Eminent Scholar Chair in Physics. Before moving to Florida in 1991, he served as director for the Institute for Theoretical Physics from 1984-1989 and was the Chancellor's Professor at the University of California in Santa Barbara from 1984-1991. -

Twenty Five Hundred Years of Small Science What’S Next?
Twenty Five Hundred Years of Small Science What’s Next? Lloyd Whitman Assistant Director for Nanotechnology White House Office of Science and Technology Policy Workshop on Integrated Nanosystems for Atomically Precise Manufacturing Berkeley, CA, August 5, 2015 Democritus (ca. 460 – 370 BC) Everything is composed of “atoms” Atomos (ἄτομος): that which can not be cut www.phil-fak.uni- duesseldorf.de/philo/galerie/antike/ demokrit.html Quantum Mechanics (1920s) Max Planck 1918* Albert Einstein 1921 Niels Bohr 1922 Louis de Broglie 1929 Max Born 1954 Paul Dirac 1933 On the Theory of Quanta Louis-Victor de Broglie Werner Heisenberg 1932 Wolfgang Pauli 1945 Erwin Schrödinger 1933 *Nobel Prizes in Physics https://tel.archives-ouvertes.fr/tel- 00006807 Ernst Ruska (1906 – 1988) Electron Microscopy Magnifying higher than the light microscope - 1933 Nobel Prize in Physics 1986 www.nobelprize.org/nobel_prizes/physics/laureates /1986/ruska-lecture.pdf Richard Feynman (1918-1988) There's Plenty of Room at the Bottom, An Invitation to Enter a New Field of Physics What would happen if we could arrange the atoms one by one the way we want them…? December 29, 1959 richard-feynman.net Heinrich Rohrer (1933 – 2013) Gerd Binnig Atomic resolution Scanning Tunneling Microscopy - 1981 1983 I could not stop looking at the images. It was like entering a new world. Gerd Binnig, Nobel lecture Binnig, et al., PRL 50, 120 (1983) Nobel Prize in Physics 1986 C60: Buckminsterfullerene Kroto, Heath, O‘Brien, Curl and September 1985 Smalley - 1985 …a remarkably stable cluster consisting of 60 carbon atoms…a truncated icosahedron. Nature 318, 162 (1985) http://www.acs.org/content/acs/en/education/whatis chemistry/landmarks/fullerenes.html Nobel Prize in Chemistry 1996 Curl, Kroto, and Smalley Positioning Single Atoms with a Scanning Tunnelling Microscope Eigler and Schweizer - 1990 …fabricate rudimentary structures of our own design, atom by atom. -

Appendix E • Nobel Prizes
Appendix E • Nobel Prizes All Nobel Prizes in physics are listed (and marked with a P), as well as relevant Nobel Prizes in Chemistry (C). The key dates for some of the scientific work are supplied; they often antedate the prize considerably. 1901 (P) Wilhelm Roentgen for discovering x-rays (1895). 1902 (P) Hendrik A. Lorentz for predicting the Zeeman effect and Pieter Zeeman for discovering the Zeeman effect, the splitting of spectral lines in magnetic fields. 1903 (P) Antoine-Henri Becquerel for discovering radioactivity (1896) and Pierre and Marie Curie for studying radioactivity. 1904 (P) Lord Rayleigh for studying the density of gases and discovering argon. (C) William Ramsay for discovering the inert gas elements helium, neon, xenon, and krypton, and placing them in the periodic table. 1905 (P) Philipp Lenard for studying cathode rays, electrons (1898–1899). 1906 (P) J. J. Thomson for studying electrical discharge through gases and discover- ing the electron (1897). 1907 (P) Albert A. Michelson for inventing optical instruments and measuring the speed of light (1880s). 1908 (P) Gabriel Lippmann for making the first color photographic plate, using inter- ference methods (1891). (C) Ernest Rutherford for discovering that atoms can be broken apart by alpha rays and for studying radioactivity. 1909 (P) Guglielmo Marconi and Carl Ferdinand Braun for developing wireless telegraphy. 1910 (P) Johannes D. van der Waals for studying the equation of state for gases and liquids (1881). 1911 (P) Wilhelm Wien for discovering Wien’s law giving the peak of a blackbody spectrum (1893). (C) Marie Curie for discovering radium and polonium (1898) and isolating radium. -

The Story of the Invention of the Scanning Tunnelling Microscope (STM)
ANNALS OF SCIENCE, Vol. 65, No. 1, January 2008, 101Á125 Searching for Asses, Finding a Kingdom: The Story of the Invention of the Scanning Tunnelling Microscope (STM) GALINA GRANEK and GIORA HON Department of Philosophy, University of Haifa, Haifa 31905, Israel. Email: [email protected]; [email protected] Received 25 October 2006. Revised paper accepted 17 May 2007 Summary We offer a novel historical-philosophical framework for discussing experimental practice which we call ‘Generating Experimental Knowledge’. It combines three different perspectives: experimental systems, concept formation, and the pivotal role of error. We then present an historical account of the invention of the Scanning Tunnelling Microscope (STM), or Raster-Tunnelmikroskop,and interpret it within the proposed framework. We show that at the outset of the STM project, Binnig and Rohrer*the inventors of the machine*filed two patent disclosures; the first is dated 22 December 1978 (Switzerland), and the second, two years later, 12 September 1980 (US). By studying closely these patent disclosures, the attempts to realize them, and the subsequent development of the machine, we present, within the framework of generating experimental knowledge, a new account of the invention of the STM. While the realization of the STM was still a long way off, the patent disclosures served as blueprints, marking the changes that had to be introduced on the way from the initial idea to its realization. Contents 1. Introduction: accounts of the invention of STM ..................102 2. A novel methodological framework: ‘Generating Experimental Knowledge’ . .........................................104 3. A new account: the three phases .............................106 3.1 Phase one: the blueprint*patent disclosures of STM. -

The First Awarding of the Heinrich Rohrer Medals
The First Awarding of The Heinrich Rohrer Medals June 2014, The Surface Science Society of Japan Masaharu Oshima, President It is our great pleasure to announce the winners of the first awarding of The Heinrich Rohrer Medals. The Medal has been established after the name of Late Dr. Heinrich Rohrer, one of the Laureates of Nobel Prize in Physics in 1986, for recognizing researchers who have made the world-top level achievements in the fields of nanoscience and nanotechnology. The Heinrich Rohrer Medal –Grand Medal– - Roland Wiesendanger (born in 1961) Professor in University of Hamburg, Germany "For his pioneering and ground-breaking achievements on spin-resolved scanning tunneling microscopy and spectroscopy, bringing about very deep insights in spin-related properties of materials at atomic scale" The Heinrich Rohrer Medal –Rising Medal– - Yoshiaki Sugimoto (born in 1978) Associate Professor in Osaka University, Japan "For his outstanding contributions to manipulation and chemical identification of individual atoms using atomic force microscopy" The Heinrich Rohrer Medal –Rising Medal– - Jan Hugo Dil (born in 1977) SNSF Professor in Ecole Polytechnique Fédérale de Lausanne, Switzerland "For his leading and creative roles in identifying novel spin structures using synchrotron radiation-based spin- and angle-resolved photoemission spectroscopy" Award Committee Members - Masaru Tsukada (Tohoku University, Japan, Committee Chair,) - Heike E. Riel (IBM Zurich, Switzerland) - Wolf-Dieter Schneider (EPFL, Switzerland) - Patrick Soukiassian (University of Paris-Sud/Orsay, France) - Flemming Besenbacher (Aarhus University, Denmark) - Michel A. Van Hove (Hong Kong Baptist University, Hong Kong), - Matthias Scheffler (Fritz Haber Institute, Germany) - Kunio Takayanagi (Tokyo Institute of Technology, Japan) Award Ceremony The award ceremony will be held at The 7th International Symposium on Surface Science, ISSS-7 (http://www.sssj.org/isss7/), on 2-6 November, 2014, at Kunibiki Messe, Shimane, Japan, which is organized by The Surface Science Society of Japan. -

INMUNOTERAPIA CONTRA EL CÁNCER ESPECIAL Inmunoterapia Contra El Cáncer
ESPECIAL INMUNOTERAPIA CONTRA EL CÁNCER ESPECIAL Inmunoterapia contra el cáncer CONTENIDO Una selección de nuestros mejores artículos sobre las distintas estrategias de inmunoterapia contra el cáncer. Las defensas contra el cáncer El científico paciente Karen Weintraub Katherine Harmon Investigación y Ciencia, junio 2016 Investigación y Ciencia, octubre 2012 Desactivar el cáncer Un interruptor Jedd D. Wolchok Investigación y Ciencia, julio 2014 para la terapia génica Jim Kozubek Investigación y Ciencia, mayo 2016 Una nueva arma contra el cáncer Viroterapia contra el cáncer Avery D. Posey Jr., Carl H. June y Bruce L. Levine Douglas J. Mahoney, David F. Stojdl y Gordon Laird Investigación y Ciencia, mayo 2017 Investigación y Ciencia, enero 2015 Vacunas contra el cáncer Inmunoterapia contra el cáncer Eric Von Hofe Lloyd J. Old Investigación y Ciencia, diciembre 2011 Investigación y Ciencia, noviembre 1996 EDITA Prensa Científica, S.A. Muntaner, 339 pral. 1a, 08021 Barcelona (España) [email protected] www.investigacionyciencia.es Copyright © Prensa Científica, S.A. y Scientific American, una división de Nature America, Inc. ESPECIAL n.o 36 ISSN: 2385-5657 En portada: iStock/royaltystockphoto | Imagen superior: iStock/man_at_mouse Takaaki Kajita Angus Deaton Paul Modrich Arthur B. McDonald Shuji Nakamura May-Britt Moser Edvard I. Moser Michael Levitt James E. Rothman Martin KarplusMÁS David DE J. 100 Wineland PREMIOS Serge Haroche NÓBEL J. B. Gurdon Adam G.han Riess explicado André K. Geim sus hallazgos Carol W. Greider en Jack W. Szostak E. H. Blackburn W. S. Boyle Yoichiro Nambu Luc MontagnierInvestigación Mario R. Capecchi y Ciencia Eric Maskin Roger D. Kornberg John Hall Theodor W. -

Heinrich Rohrer (1933–2013) Co-Inventor of the Scanning Tunnelling Microscope
COMMENT OBITUARY Heinrich Rohrer (1933–2013) Co-inventor of the scanning tunnelling microscope. einrich Rohrer, Heini to those who contemplate a new device. By 1981, the eventually verified by other groups and knew him, helped to open the door pair had designed the world’s first scanning presented at a workshop on the STM in the to nanotechnology. With Gerd tunnelling microscope (STM). Austrian Alps in 1985. Devices such as the HBinnig, he produced a device that allowed Unlike conventional microscopes, the atomic force microscope (AFM) — a very researchers to image and measure atoms STM did not use lenses. Instead, a probe high resolution type of scanning microscope and molecules, and to manipulate them. sharpened to a single atom at the tip was that measures the atomic forces between the Rohrer, who died on 16 May, tip of a probe and the surface being three weeks before his 80th scanned — have their roots in this birthday, was born in 1933, half meeting. During the last night of an hour after his twin sister. He the workshop, the mountains were grew up in the village of Buchs in abuzz with crazy ideas about how eastern Switzerland. Rohrer stud- such microscopes might be used ied physics at the Swiss Federal in applications in all sorts of fields, Institute of Technology (ETH) from fundamental physics and — ZURICH IBM RESEARCH in Zurich, where he remained to chemistry to information tech- pursue a PhD. It was during his nology, quantum computing and PhD years that he first came into molecular electronics, as well as in contact with the nanometre scale, the life sciences. -

John Robert Schrieffer Daniel Arovas, Greg Boebinger, and Nick Bonesteel
John Robert Schrieffer Daniel Arovas, Greg Boebinger, and Nick Bonesteel Citation: Physics Today 73, 1, 63 (2020); doi: 10.1063/PT.3.4395 View online: https://doi.org/10.1063/PT.3.4395 View Table of Contents: https://physicstoday.scitation.org/toc/pto/73/1 Published by the American Institute of Physics ARTICLES YOU MAY BE INTERESTED IN Gaurang Bhaskar Yodh Physics Today 73, 64 (2020); https://doi.org/10.1063/PT.3.4396 Johannes Kepler’s pursuit of harmony Physics Today 73, 36 (2020); https://doi.org/10.1063/PT.3.4388 Rare earths in a nutshell Physics Today 73, 66 (2020); https://doi.org/10.1063/PT.3.4397 The sounds around us Physics Today 73, 28 (2020); https://doi.org/10.1063/PT.3.4387 Charles Kittel Physics Today 72, 73 (2019); https://doi.org/10.1063/PT.3.4326 The usefulness of GRE scores Physics Today 73, 10 (2020); https://doi.org/10.1063/PT.3.4376 OBITUARIES made when Cooper solved the problem John Robert Schrieffer of two electrons above a quiescent Fermi towering figure in theoretical con- sea. He took into account the effective at- densed-matter physics, John Robert tractive interaction mediated by phonons, ASchrieffer died on 27 July 2019 in Tal- which resulted in a bound state of elec- lahassee, Florida. He is best known for trons. Schrieffer’s focus crystallized on his crucial contributions to the theory of finding a many-electron theory that superconductivity, a problem that since could incorporate Cooper’s bound pairs, its discovery in 1911 had vexed physi- which, though not quite bosons, some- cists searching for a microscopic expla- how needed to be condensed. -

K. Alex Müller Nobel Prize in Physics 1987
K. Alex Müller Nobel Prize in Physics 1987 is convinced that he at last has a water- no one took him seriously. “Exactly tight explanation for the phenomenon that motivated me. I wanted to swim that he and J. Georg Bednorz discovered against the current.” 28 years ago: High-temperature super- He says that he owes his persistence conductivity in copper oxides. This and his desire to think outside the box should bring a decades-long dispute to his childhood, which was not easy, to a happy end, at least from Müller’s as Müller explains. The son of a sales- perspective. With his explanation, how- man and grandson of a chocolate man- ever, Müller has launched a new debate ufacturer, Karl Alex spent part of his on the distribution of matter in the uni- childhood in Lugano. After the early verse. But more of that later. death of his mother, when he was just Erice, Sicily, summer 1983: K. Alex eleven, he went to boarding school in Müller is sitting on a bench in the cas- Schiers. Holidays were spent with his Nobel Prize in Physics 1987 “for the tle grounds and enjoying the view. As pioneering discovery of super- he gazes into the distance, his mind K. Alex Müller was 56 when conductivity in ceramic materials” buzzes with ideas. He had just listened he decided to take on a new to a lecture by Harry Thomas which * 20 April 1927 in Basel challenge – researching dealt with the possible existence of superconductors. 1962–1970 Privatdozent Jahn-Teller polarons – “quasi-parti- 1970–1987 Adjunct Professor cles” that occur when electrons move 1987–1994 Professor of Solid-State Physics through a crystal lattice. -

Vacuum Science & Technology Timeline
1972 – 1975 First oil-free piston Johan K. Fremerey vacuum pump Spinning rotor John L. Farrant vacuum gauge 1974 1972 Low-Pressure Chemical Vapor Deposition of Silicon Dioxide from Tetraethoxysilane Dan L. Burt, Richard F. Taraci and John E. Zavion U.S. Patent 3934060 (1976), filed 1973 Altair 8800 Computer 1975 Structure Zone Model for sputter-deposited films John A. Thornton 1974 R. C. Merrill G.J. Egan, B.W. Paszek and A.J. Aronson Roll coater for deposition on Ferrofluidic™ rotary shaft seal plastic film Reactive ion etching Ferrofluidics Corp. 1972 Horuhiko Abe, Japan Steven Yoneo Muto 1974 Plasma etching of semiconductors U.S. Patent 3971684 (1976) U.S. Patent 3880684 (1975) filed 1973 filed 1973 Kalrez ® perfluorocarbon elastomers E. I. du Pont de Nemours & Co. c. 1975 Last Apollo Mission Cryo-pumps for The Earth in the vacuum Space Simulation and of space – from Apollo-17 semiconductor fabrication (NASA - 1972) John S. Chapin 1975 Planar magnetron sputter Low-Pressure Chemical Deposition deposition source Special Report: Vacuum of Polysilicon U.S. Patent 4166018 (1979 ) Zenith shuts down Physics Today Jerry L. Kruma and Paul G. Hilton filed 1974 Lansdale, PA unit August 1972 U.S. Patent 3900597 (1975) 1975 filed 1973 1972 1975 Vacuum Science & Technology Timeline Kai Manne Börje Siegbahn Nicolaas Bloembergen Ernst August Friedrich Ruska 1976 – 1989 (1919–1981) and Arthur L. Schawlow (1906-1988) Nobel Prize in Physics Nobel Prize in Physics Nobel Prize for transmission for high resolution for laser spectroscopy electron microscope electron spectroscopy 1981 1981 1986 Wolfgang Paul Gerd Binnig and Heinrich Rohrer Nobel Prize in Physics Nobel Prize in Physics for for Paul Trap for scanning tunneling microscopy Intel 8086 charged particles 1986 Viking I and II 16-bit microprocessor. -

The Third Awarding of the Heinrich Rohrer Medals
The Third Awarding of The Heinrich Rohrer Medals February 1, 2020 The Japan Society of Vacuum and Surface Science (JVSS) It is our great pleasure to announce the winners of the third awarding of The Heinrich Rohrer Medals. The Medal was established after the name of Late Dr. Heinrich Rohrer, one of the Laureates of Nobel Prize in Physics in 1986, for recognizing researchers who have made the world-top level achievements in the fields of nanoscience and nanotechnology. The 3rd Heinrich Rohrer Medal –Grand Medal– - Andreas Heinrich Ewha Womans University, Korea "For his ground-breaking development of scanning tunneling microscope methods to st udy the spin properties of magnetic atoms on surfaces for revealing the quantum nature of the magnetism at the atomic scale" The 3rd Heinrich Rohrer Medal –Rising Medal– - Takashi Kumagai (born in 1984) Fritz Haber Institute of the Max Planck Society, Germany " For his outstanding achievements in the field of near-field physics and chemistry in plasmonic STM junctions " Award Ceremony The award ceremony will be held at The 9th International Symposium on Surface Science, ISSS-9 (https://www.jvss.jp/isss9/), on November 15-19, 2020, at Sunport Takamatsu Convention Center, Takamatsu, Kagawa, Japan, which is organized by The Japan Society of Vacuum and Surface Science (JVSS). The awarding will be in collaboration with Swiss Embassy in Japan. The laureates will deliver the award lectures on their research achievements during ISSS-9. Operated with - IBM Research-Zurich Cosponsored by - JEOL Ltd. - Scienta Omicron, Inc. - UNISOKU Co., Ltd. Award Achievements The 3rd Heinrich Rohrer Medal –Grand Medal– Prof. -
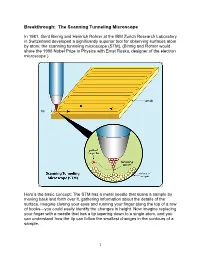
Breakthrough: the Scanning Tunneling Microscope
Breakthrough: The Scanning Tunneling Microscope In 1981, Gerd Binnig and Heinrich Rohrer at the IBM Zurich Research Laboratory in Switzerland developed a significantly superior tool for observing surfaces atom by atom: the scanning tunneling microscope (STM). (Binnig and Rohrer would share the 1986 Nobel Prize in Physics with Ernst Ruska, designer of the electron microscope.) Here’s the basic concept: The STM has a metal needle that scans a sample by moving back and forth over it, gathering information about the details of the surface. Imagine closing your eyes and running your finger along the top of a row of books—you could easily identify the changes in height. Now imagine replacing your finger with a needle that has a tip tapering down to a single atom, and you can understand how the tip can follow the smallest changes in the contours of a sample. 1 The needle doesn’t touch the sample, however, but stays about the width of two atoms above it. The STM takes advantage of what’s called the tunnel effect: If a voltage is applied to the tiny distance between the needle and the sample, electrons are able to tunnel, or jump, between the needle and the sample, creating an electric current. A computer receives the electrical signal and directs the needle to move up or down to keep the current constant—which keeps the distance between needle and sample constant. The path of the needle is recorded, and the computer can display that information as a grayscale image or topographical map. Scientists can add color to make the image easier to interpret.