Resolving Titin's Lifecycle and the Spatial Organization of Protein
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of the State of Infrared Detectors for Astronomy in Retrospect of the June 2002 Workshop on Scientific Detectors for Astronomy
Review of the state of infrared detectors for astronomy in retrospect of the June 2002 Workshop on Scientific Detectors for Astronomy. Gert Fingera and James W. Beleticb a European Southern Obseravatory, D-85748 Garching, Germany b W. M. Keck Observatory 65-1120 Mamalahoa Hwy., Kamuela, Hi 96743, USA ABSTRACT Only two months ago, in June 2002, a workshop on scientific detectors for astronomy was held in Waimea, where for the first time both experts on optical CCD’s and infrared detectors working at the cutting edge of focal plane technology gathered. An overview of new developments in optical detectors such as CCD’s and CMOS devices will be given elsewhere in these proceedings [1]. This paper will focus on infrared detector developments carried out at the European Southern Observatory ESO and will also include selected highlights of infrared focal plane technology as presented at the Waimea workshop. Three main detector developments for ground based astronomers are currently pushing infrared focal plane technology. In the near infrared from 1 to 5 mm two technologies, both aiming for buttable 2Kx2K mosaics, will be reviewed, namely InSb and HgCdTe grown by LPE or MBE on Al2O3, Si or CdZnTe substrates. Blocked impurity band Si:As arrays cover the mid infrared spectral range from 8 to 28 mm. Since the video signal of infrared arrays, contrary to CCD’s, is DC coupled, long exposures with IR arrays are extremely susceptible to drifts and low frequency noise pick- up down to the mHz regime. New techniques to reduce thermal drifts and suppress low frequency noise with on-chip reference pixels will be discussed. -

High Frequency (HF)
Calhoun: The NPS Institutional Archive Theses and Dissertations Thesis Collection 1990-06 High Frequency (HF) radio signal amplitude characteristics, HF receiver site performance criteria, and expanding the dynamic range of HF digital new energy receivers by strong signal elimination Lott, Gus K., Jr. Monterey, California: Naval Postgraduate School http://hdl.handle.net/10945/34806 NPS62-90-006 NAVAL POSTGRADUATE SCHOOL Monterey, ,California DISSERTATION HIGH FREQUENCY (HF) RADIO SIGNAL AMPLITUDE CHARACTERISTICS, HF RECEIVER SITE PERFORMANCE CRITERIA, and EXPANDING THE DYNAMIC RANGE OF HF DIGITAL NEW ENERGY RECEIVERS BY STRONG SIGNAL ELIMINATION by Gus K. lott, Jr. June 1990 Dissertation Supervisor: Stephen Jauregui !)1!tmlmtmOlt tlMm!rJ to tJ.s. eave"ilIE'il Jlcg6iielw olil, 10 piolecl ailicallecl",olog't dU'ie 18S8. Btl,s, refttteste fer litis dOCdiii6i,1 i'lust be ,ele"ed to Sapeihil6iiddiil, 80de «Me, "aial Postg;aduulG Sclleel, MOli'CIG" S,e, 98918 &988 SF 8o'iUiid'ids" PM::; 'zt6lI44,Spawd"d t4aoal \\'&u 'al a a,Sloi,1S eai"i,al'~. 'Nsslal.;gtePl. Be 29S&B &198 .isthe 9aleMBe leclu,sicaf ,.,FO'iciaKe" 6alite., ea,.idiO'. Statio", AlexB •• d.is, VA. !!!eN 8'4!. ,;M.41148 'fl'is dUcO,.Mill W'ilai.,s aliilical data wlrose expo,l is idst,icted by tli6 Arlil! Eurse" SSPItial "at FRIis ee, 1:I.9.e. gec. ii'S1 sl. seq.) 01 tlls Exr;01l ftle!lIi"isllatioli Act 0' 19i'9, as 1tI'I'I0"e!ee!, "Filill ell, W.S.€'I ,0,,,,, 1i!4Q1, III: IIlIiI. 'o'iolatioils of ltrese expo,lla;;s ale subject to 960616 an.iudl pSiiaities. -

Regulation of Titin-Based Cardiac Stiffness by Unfolded Domain Oxidation (Undox)
Regulation of titin-based cardiac stiffness by unfolded domain oxidation (UnDOx) Christine M. Loeschera,1, Martin Breitkreuzb,1, Yong Lia, Alexander Nickelc, Andreas Ungera, Alexander Dietld, Andreas Schmidte, Belal A. Mohamedf, Sebastian Kötterg, Joachim P. Schmitth, Marcus Krügere,i, Martina Krügerg, Karl Toischerf, Christoph Maackc, Lars I. Leichertj, Nazha Hamdanib, and Wolfgang A. Linkea,2 aInstitute of Physiology II, University of Munster, 48149 Munster, Germany; bInstitute of Physiology, Ruhr University Bochum, 44801 Bochum, Germany; cComprehensive Heart Failure Center Wuerzburg, University Clinic Wuerzburg, 97078 Wuerzburg, Germany; dDepartment of Internal Medicine II, University Hospital Regensburg, 93053 Regensburg, Germany; eInstitute for Genetics, University of Cologne, 50931 Cologne, Germany; fDepartment of Cardiology and Pneumology, University Medicine Goettingen, 37075 Goettingen, Germany; gDepartment of Cardiovascular Physiology, Heinrich Heine University, 40225 Düsseldorf, Germany; hDepartment of Pharmacology and Clinical Pharmacology, Heinrich Heine University, 40225 Düsseldorf, Germany; iCenter for Molecular Medicine and Excellence Cluster "Cellular Stress Responses in Aging-Associated Diseases" (CECAD), University of Cologne, 50931 Cologne, Germany; and jInstitute for Biochemistry and Pathobiochemistry, Ruhr University Bochum, 44801 Bochum, Germany Edited by Jonathan Seidman, Harvard University, Boston, MA, and approved August 12, 2020 (received for review March 14, 2020) The relationship between oxidative stress and -

AN EMPIRICAL DETERMINATION of the INTERGALACTIC BACKGROUND LIGHT USING NEAR-INFRARED DEEP GALAXY SURVEY DATA out to 5 Μm and the GAMMA-RAY OPACITY of the UNIVERSE
The Astrophysical Journal, 784:138 (6pp), 2014 April 1 doi:10.1088/0004-637X/784/2/138 C 2014. The American Astronomical Society. All rights reserved. Printed in the U.S.A. AN EMPIRICAL DETERMINATION OF THE INTERGALACTIC BACKGROUND LIGHT USING NEAR-INFRARED DEEP GALAXY SURVEY DATA OUT TO 5 μm AND THE GAMMA-RAY OPACITY OF THE UNIVERSE Sean T. Scully1, Matthew A. Malkan2, and Floyd W. Stecker2,3 1 Department of Physics and Astronomy, James Madison University, Harrisonburg, VA 22807, USA 2 Department of Physics and Astronomy, University of California, Los Angeles, CA 90095, USA; [email protected] 3 Astrophysics Science Division, NASA/Goddard Space Flight Center, 8800 Greenbelt Road, Greenbelt, MD 20771, USA Received 2014 January 21; accepted 2014 February 24; published 2014 March 14 ABSTRACT We extend our previous model-independent determination of the intergalactic background light, based purely on galaxy survey data, out to a wavelength of 5 μm. Our approach enables us to constrain the range of photon densities, based on the uncertainties from observationally determined luminosity densities and colors. We further determine a 68% confidence upper and lower limit on the opacity of the universe to γ -rays up to energies of 1.6/(1 + z)TeV. A comparison of our lower limit redshift-dependent opacity curves to the opacity limits derived from the results of both ground-based air Cerenkov telescope and Fermi-LAT observations of PKS 1424+240 allows us to place a new upper limit on the redshift of this source, independent of IBL modeling. Key words: BL Lacertae objects: individual (PKS 1424+240) – diffuse radiation – gamma rays: general Online-only material: color figure <z< 1. -

Postmortem Changes in the Myofibrillar and Other Cytoskeletal Proteins in Muscle
BIOCHEMISTRY - IMPACT ON MEAT TENDERNESS Postmortem Changes in the Myofibrillar and Other C'oskeletal Proteins in Muscle RICHARD M. ROBSON*, ELISABETH HUFF-LONERGAN', FREDERICK C. PARRISH, JR., CHIUNG-YING HO, MARVIN H. STROMER, TED W. HUIATT, ROBERT M. BELLIN and SUZANNE W. SERNETT introduction filaments (titin), and integral Z-line region (a-actinin, Cap Z), as well as proteins of the intermediate filaments (desmin, The cytoskeleton of "typical" vertebrate cells contains paranemin, and synemin), Z-line periphery (filamin) and three protein filament systems, namely the -7-nm diameter costameres underlying the cell membrane (filamin, actin-containing microfilaments, the -1 0-nm diameter in- dystrophin, talin, and vinculin) are listed along with an esti- termediate filaments (IFs), and the -23-nm diameter tubu- mate of their abundance, approximate molecular weights, lin-containing microtubules (Robson, 1989, 1995; Robson and number of subunits per molecule. Because the myofibrils et al., 1991 ).The contractile myofibrils, which are by far the are the overwhelming components of the skeletal muscle cell major components of developed skeletal muscle cells and cytoskeleton, the approximate percentages of the cytoskel- are responsible for most of the desirable qualities of muscle eton listed for the myofibrillar proteins (e.g., myosin, actin, foods (Robson et al., 1981,1984, 1991 1, can be considered tropomyosin, a-actinin, etc.) also would represent their ap- the highly expanded corollary of the microfilament system proximate percentages of total myofibrillar protein. of non-muscle cells. The myofibrils, IFs, cell membrane skel- eton (complex protein-lattice subjacent to the sarcolemma), Some Important Characteristics, Possible and attachment sites connecting these elements will be con- Roles, and Postmortem Changes of Key sidered as comprising the muscle cell cytoskeleton in this Cytoskeletal Proteins review. -

Α-Actinin/Titin Interaction: a Dynamic and Mechanically Stable Cluster of Bonds in the Muscle Z-Disk
α-Actinin/titin interaction: A dynamic and mechanically stable cluster of bonds in the muscle Z-disk Marco Grisona, Ulrich Merkela, Julius Kostanb, Kristina Djinovic-Carugob,c, and Matthias Riefa,d,1 aPhysik Department E22, Technische Universität München, 85748 Garching, Germany; bDepartment of Structural and Computational Biology, Max F. Perutz Laboratories, University of Vienna, A-1030 Vienna, Austria; cDepartment of Biochemistry, Faculty of Chemistry and Chemical Technology, University of Ljubljana, SI-1000 Ljubljana, Slovenia; and dMunich Center for Integrated Protein Science, 81377 Munich, Germany Edited by James A. Spudich, Stanford University School of Medicine, Stanford, CA, and approved December 16, 2016 (received for review August 2, 2016) Stable anchoring of titin within the muscle Z-disk is essential for In humans, the isoforms of titin exhibit four to seven Z-repeats preserving muscle integrity during passive stretching. One of the (15, 16, 20). The structure of the EF3-4 hands complex with titin main candidates for anchoring titin in the Z-disk is the actin cross- Z-repeat 7 shows the bound Z-repeat in an α-helical confor- linker α-actinin. The calmodulin-like domain of α-actinin binds to mation (21). In solution assays, binding affinities of various the Z-repeats of titin. However, the mechanical and kinetic prop- Z-repeats to EF3-4 were determined to lie in the micromolar erties of this important interaction are still unknown. Here, we use range (22). Micromolar affinity points only to a moderately a dual-beam optical tweezers assay to study the mechanics of this stable interaction, the kinetics of which are unknown. -

Absolute Radiant Power Measurement for the Au M Lines of Laser-Plasma Using a Calibrated Broadband Soft X-Ray Spectrometer with Flat-Spectral Response Ph
Absolute radiant power measurement for the Au M lines of laser-plasma using a calibrated broadband soft X-ray spectrometer with flat-spectral response Ph. Troussel, B. Villette, B. Emprin, G. Oudot, V. Tassin, F. Bridou, Franck Delmotte, M. Krumrey To cite this version: Ph. Troussel, B. Villette, B. Emprin, G. Oudot, V. Tassin, et al.. Absolute radiant power measurement for the Au M lines of laser-plasma using a calibrated broadband soft X-ray spectrometer with flat- spectral response. Review of Scientific Instruments, American Institute of Physics, 2014, 85 (1), pp.013503. 10.1063/1.4846915. hal-01157538 HAL Id: hal-01157538 https://hal.archives-ouvertes.fr/hal-01157538 Submitted on 16 Nov 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW OF SCIENTIFIC INSTRUMENTS 85, 013503 (2014) Absolute radiant power measurement for the Au M lines of laser-plasma using a calibrated broadband soft X-ray spectrometer with flat-spectral response Ph. Troussel,1 B. Villette,1 B. Emprin,1,2 G. Oudot,1 V. Tassin,1 F. Bridou,2 F. Delmotte,2 and M. Krumrey3 1CEA/DAM/DIF, Bruyères le Châtel, 91297 Arpajon, France 2Laboratoire Charles Fabry, Institut d’Optique, CNRS, University Paris-Sud, 2, Avenue Augustin Fresnel, RD128, 91127 Palaiseau Cedex, France 3Physikalisch-Technische Bundesanstalt (PTB), Abbestr. -
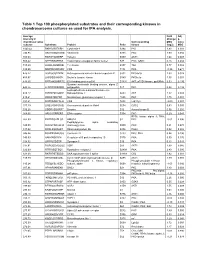
Table 1 Top 100 Phosphorylated Substrates and Their Corresponding Kinases in Chondrosarcoma Cultures As Used for IPA Analysis
Table 1 Top 100 phosphorylated substrates and their corresponding kinases in chondrosarcoma cultures as used for IPA analysis. Average Fold Adj intensity in Change p- chondrosarcoma Corresponding MSC value cultures Substrate Protein Psite kinase (log2) MSC 1043.42 RKKKVSSTKRH Cytohesin-1 S394 PKC 1.83 0.001 746.95 RKGYRSQRGHS Vitronectin S381 PKC 1.00 0.056 709.03 RARSTSLNERP Tuberin S939 AKT1 1.64 0.008 559.42 SPPRSSLRRSS Transcription elongation factor A-like1 S37 PKC; GSK3 0.18 0.684 515.29 LRRSLSRSMSQ Telethonin S157 Titin 0.77 0.082 510.00 MQPDNSSDSDY CD5 T434 PKA -0.35 0.671 476.27 GGRGGSRARNL Heterogeneous nuclear ribonucleoprotein K S302 PKCdelta 1.03 0.028 455.97 LKPGSSHRKTK Bruton's tyrosine kinase S180 PKCbeta 1.55 0.001 444.65 RRRMASMQRTG E1A binding protein p300 S1834 AKT; p70S6 kinase; pp90Rsk 0.53 0.195 Guanine nucleotide binding protein, alpha Z 440.26 HLRSESQRQRR polypeptide S27 PKC 0.88 0.199 6-phosphofructo-2-kinase/fructose-2,6- 424.12 RPRNYSVGSRP biphosphatase 2 S483 AKT 1.32 0.003 419.61 KKKIATRKPRF Metabotropic glutamate receptor 1 T695 PKC 1.75 0.001 391.21 DNSSDSDYDLH CD5 T453 Lck; Fyn -2.09 0.001 377.39 LRQLRSPRRAQ Ras associated protein Rab4 S204 CDC2 0.63 0.091 376.28 SSQRVSSYRRT Desmin S12 Aurora kinase B 0.56 0.255 369.05 ARIGGSRRERS EP4 receptor S354 PKC 0.29 0.543 RPS6 kinase alpha 3; PKA; 367.99 EPKRRSARLSA HMG14 S7 PKC -0.01 0.996 Peptidylglycine alpha amidating 349.08 SRKGYSRKGFD monooxygenase S930 PKC 0.21 0.678 347.92 RRRLSSLRAST Ribosomal protein S6 S236 PAK2 0.02 0.985 346.84 RSNPPSRKGSG Connexin -

Association of Titin and Myosin Heavy Chain in Developing Skeletal Muscle (Myogenesis/Cytoskeleton/Assembly in Vvo) W
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 74%-7500, August 1992 Cell Biology Association of titin and myosin heavy chain in developing skeletal muscle (myogenesis/cytoskeleton/assembly in vvo) W. B. ISAACS*, I. S. KIM, A. STRUVE, AND A. B. FULTONt Department of Biochemistry, University of Iowa, Iowa City, IA 52242 Communicated by Sheldon Penman, May 22, 1992 ABSTRACT To understand molecular interactions that deficient medium (10). After labeling, cultures either were organize developing myoflbrils, we examined the biosynthesis extracted immediately or were chased by adding complete and interaction of titin and myosin heavy chain in cultures of medium supplemented with 2 mM unlabeled methionine and developing muscle. Use of pulse-labeling, immunoprecipita- incubating at 370C for various times before extraction. Ex- tion, and a reversible cross-linking procedure demonstrates tractions used 0.5% Triton X-100 in extraction buffer (100 that within minutes of synthesis, titin and myosin heavy chain mM KCI/10 mM Pipes, pH 6.8/300 mM sucrose/2 mM can be chemically cross-linked into very large, detergent- MgCI2/1 mM EGTA) containing protease inhibitors (1 mM resistant complexes retaining many features of intact myo- phenylmethylsulfonyl fluoride and 100 mM leupeptin; ref. tubes. These complexes, predominantly of titin and myosin, 11). occur very early in myofibrillogenesis as well as later. These Immunoprecipitation. Titin was precipitated by using a data suggest that synthesis and assembly oftitin and myosin are mouse monoclonal antibody (mAb), AMF-1, as described temporally and spatially coordinated in nascent myofibrils and (10). Muscle-specific myosin heavy chain (hereafter myosin) support the hypothesis that titin molecules help to organize was precipitated with mAb MF-20 (12), a gift ofD. -

Recent Studies on Hohlraum Energitics and Hohlraum Design
Recent studies on hohlraum energitics and hohlraum design Ke Lan, Wenyi Huo, Yongsheng Li, Guoli Ren, Xin Li, Xujun Meng, Changshu Wu, Siyang Zou, Xiumei Qiao, Peijun Gu, Wudi Zheng, Dongxian Lai, and Tinggui Feng Institute of Applied Physics and Computational Mathematics, P.O. Box 8009-14 Beijing, 100088, People’s Republic of China, E-mail: [email protected] Abstract In this paper, some recent studies on hohlraum physics at IAPCM (Institute of Applied Physics and Computational Mathematics) were presented, mainly including simulation study on hohlraum physics experiments on SGIII prototype, a novel method for simultaneously determining the maximum radiation temperature and M-band fraction inside a hohlraum, the design of Au + U + Au sandwich hohlraum for 300eV ignition target, and the way to give an initial design of hohlraum size and pertinent drive laser power in order to generate a required radiation profile. 1. Introduction Hohlraum plays a key role in the study of indirect drive inertial fusion, which converts the shaped laser pulse into a symmetrically time-dependent thermal radiation to assure a nearly isentropic compression of capsule to achieve thermonuclear ignition 1-6. Hence, worldwide IFE (Inertial Fusion Energy) scientists have been paying much efforts in the study of hohlraum physics 7-11, and a series of theory for hohlraum have been developed, such as the hohlraum coupling efficiency theory, capsule radiation uniformity theory 5, and plasma-filling model 12 . Inertial Fusion program began in China in 1993. We have laser facilities of SGII in Shanghai and SGIII prototype in Mianyang 13-15 . IAPCM in Beijing mainly engages in simulation and theoretical study of Inertial Fusion. -

Titin N2A Domain and Its Interactions at the Sarcomere
International Journal of Molecular Sciences Review Titin N2A Domain and Its Interactions at the Sarcomere Adeleye O. Adewale and Young-Hoon Ahn * Department of Chemistry, Wayne State University, Detroit, MI 48202, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-(313)-577-1384 Abstract: Titin is a giant protein in the sarcomere that plays an essential role in muscle contraction with actin and myosin filaments. However, its utility goes beyond mechanical functions, extending to versatile and complex roles in sarcomere organization and maintenance, passive force, mechanosens- ing, and signaling. Titin’s multiple functions are in part attributed to its large size and modular structures that interact with a myriad of protein partners. Among titin’s domains, the N2A element is one of titin’s unique segments that contributes to titin’s functions in compliance, contraction, structural stability, and signaling via protein–protein interactions with actin filament, chaperones, stress-sensing proteins, and proteases. Considering the significance of N2A, this review highlights structural conformations of N2A, its predisposition for protein–protein interactions, and its multiple interacting protein partners that allow the modulation of titin’s biological effects. Lastly, the nature of N2A for interactions with chaperones and proteases is included, presenting it as an important node that impacts titin’s structural and functional integrity. Keywords: titin; N2A domain; protein–protein interaction 1. Introduction Citation: Adewale, A.O.; Ahn, Y.-H. The complexity of striated muscle is defined by the intricate organization of its com- Titin N2A Domain and Its ponents [1]. The involuntary cardiac and voluntary skeletal muscles are the primary types Interactions at the Sarcomere. -

The Effect of Gamma Radiation on the Conductivity of Sodium Chloride Michael Claudewell Jon Carlson Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1968 The effect of gamma radiation on the conductivity of sodium chloride Michael Claudewell Jon Carlson Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Nuclear Engineering Commons Recommended Citation Carlson, Michael Claudewell Jon, "The effect of gamma radiation on the conductivity of sodium chloride " (1968). Retrospective Theses and Dissertations. 3723. https://lib.dr.iastate.edu/rtd/3723 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been microiihned exactly as received 69-4221 CARLSON, Michael Claudewell Jon, 1939- THE EFFECT OF GAMMA RADIATION ON THE CONDUCTIVITY OF SODIUM CHLORIDE. Iowa State University, Ph.D., 1968 Engineering, nuclear University Microfilms, Inc., Ann Arbor, Michigan THE SFFECT OF GAMMA RADIATION ON THE CONDUCTIVITY OF SODIUM CHLORIDE by Michael Claudewell Jon Carlson A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Nuclear Engineering Approved; Signature was redacted for privacy. Signature was redacted for privacy.