Annual Report 2019 Cantargia AB (Publ.) 556791-6019 TABLE of CONTENTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I Nafed by City
- t, THURSDAY EDITION eparations Complete For District me Demonstration Meetin OFFICIAL NEWSPAPER OF LAMB COUNTY Dr. Ross Calvin I A Ml rft II hITV I CA f JT O Of Clovis To Be Principal Speaker C. Chesher and other Plains i 1 from the South VOLUME XXVII. LITTLEFIELD, LAMB COUNTY, TEXAS, before THURSDAY, APRIL 20, 1950 NUMBER 16 indie appearing About 300 H. D. Interior.lnsular Affairs-te- e were n Washington Club Members Are concerning a favorable ithe subcomlttee on the 23-2- Expected To Attend River Develop- - 9 lanadlan April By Desl an- City complete nafed Plans are for the bcommlttee seemed Im-th- e nual district 2 convention of tho evidence presented Women's Home Demonstration delegation favoring the Clubs which w.ll be held In thn project. Reclamation C I First Baptist Church on Tuesday,. hearing gave As April 25. ured at the Chamber Of fnmerce Week nJ cost of 11.6 cents per ' Principal speaker will bo Dr. ns cost is of afr the Ross Calvin, sector of St. Jametf In having Open House To Be j Interested Bula High Juniors Entertain Seniors At Banquet Farmer Arrested id In' from n Cnnadlnn hich would be benefit--. Observed Tuesday On Rape Charge water supply besides Lubbock, Amnrillo. re C. P. Parker, Spade srger. Plalnvlew, Level-iue- d farmer Is being held In the Lamb On Uak Page) By Booster Group SKLu, . LyHMBi? ' iMIKKt ilu!m3 . County Jail here inder $10,000 itSml bond on charges of rape and Incest PaifTBy Garden Club To Involving his daughter Ms Parker was arraigned before Serve Refreshments Justice of Peaee S. -

Foreign Direct Investment in Latin America and the Caribbean Alicia Bárcena Executive Secretary
2010 Foreign Direct Investment in Latin America and the Caribbean Alicia Bárcena Executive Secretary Antonio Prado Deputy Executive Secretary Mario Cimoli Chief Division of Production, Productivity and Management Ricardo Pérez Chief Documents and Publications Division Foreign Direct Investment in Latin America and the Caribbean, 2010 is the latest edition of a series issued annually by the Unit on Investment and Corporate Strategies of the ECLAC Division of Production, Productivity and Management. It was prepared by Álvaro Calderón, Mario Castillo, René A. Hernández, Jorge Mario Martínez Piva, Wilson Peres, Miguel Pérez Ludeña and Sebastián Vergara, with assistance from Martha Cordero, Lucía Masip Naranjo, Juan Pérez, Álex Rodríguez, Indira Romero and Kelvin Sergeant. Contributions were received as well from Eduardo Alonso and Enrique Dussel Peters, consultants. Comments and suggestions were also provided by staff of the ECLAC subregional headquarters in Mexico, including Hugo Beteta, Director, and Juan Carlos Moreno-Brid, Juan Alberto Fuentes, Claudia Schatan, Willy Zapata, Rodolfo Minzer and Ramón Padilla. ECLAC wishes to express its appreciation for the contribution received from the executives and officials of the firms and other institutions consulted during the preparation of this publication. Chapters IV and V were prepared within the framework of the project “Inclusive political dialogue and exchange of experiences”, carried out jointly by ECLAC and the Alliance for the Information Society (@lis 2) with financing from the European -

Daftar Isi Table of Content
ANALISA & PEMAHAMAN TATA KELOLA PERUSAHAAN YANG BAIK TANGGUNG JAWAB SOSIAL MANAJEMEN good corporate governance PERUSAHAAN management discussion & anlysis Corporate Sosial Responsibility DAFTAR ISI TABLE OF CONTENT PENJELASAN TEMA 2 SPLASH PAGE EXECUTIVE CHAIRMAN EXECUTIVE CHAIRMAN 4 KINERJA TAHUN 2019 2019 PERFORMANCE 7 LAPORAN MANAJEMEN MANAGEMENT REPORT 13 PROFIL PERUSAHAAN COMPANY PROFILE 27 SUMBER DAYA MANUSIA HUMAN RESOUCES 64 ANALISIS DAN PEMBAHASAN MANAJEMEN MANAGEMENT DISCUSSION AND 72 ANALYSIS TATA KELOLA PERUSAHAAN YANG BAIK GOOD CORPORATE 83 GOVERNANCE TANGGUNG JAWAB SOSIAL CORPORATE SOSIAL RESPONSIBILITY 156 ANNUAL REPORT 2019 www.mncvision.id 1 KINERJA 2019 LAPORAN MANAJEMEN PROFIL PERUSAHAAN SUMBERDAYA MANUSIA 2019 Performance Management Report Company Profile Humant Resource PENJELASAN TEMA Theme Explanation 2 PT MNC Sky Vision Tbk LAPORAN TAHUNAN 2019 ANALISA & PEMAHAMAN TATA KELOLA PERUSAHAAN YANG BAIK TANGGUNG JAWAB SOSIAL MANAJEMEN good corporate governance PERUSAHAAN management discussion & anlysis Corporate Sosial Responsibility PT MNC Sky Vision Tbk berhasil melalui ketidakpastian PT MNC Sky Vision Tbk managed to survive within ekonomi serta tantangan-tantangan yang datang dari the economic uncertainties and the challenges kondisi eksternal sepanjang tahun 2019. Kinerja seluruh originating from external conditions throughout 2019. sektor bisnis termasuk industri TV berlangganan The performance of all business sectors, including Pay yang terhambat justru membuka kesempatan bagi TV industry, that is currently being hampered, provides Perseroan untuk mewujudkan berbagai perbaikan an opportunity for the Company to advance, work serta bekerja lebih keras dan cerdas. harder, and smarter. Semangat transformasi diinisiasi dan memacu seluruh The spirit of transformation spurred all of the elemen Perseroan untuk mengembangkan peluang Company’s elements to develop innovative business bisnis yang inovatif. -

PT MNC VISION NETWORKS Tbk
INVESTMENT TEASER INITIAL PUBLIC OFFERING PT MNC VISION NETWORKS Tbk. A. RISALAH INVESTASI Perseroan Bersama Dengan Entitas Anak Merupakan Market Leader Dalam Industri Media Di Indonesia. Perseroan bersama Entitas Anak yaitu MNC Vision menjadi market leader dalam bisnis TV berlangganan DTH dengan pangsa pasar 96% dan MNC Play merupakan Perusahaan No.3 terbesar di industri fixed broadband/IPTV. Perseroan bersama Entitas Anak memiliki basis pelanggan gabungan DTH dan fixed broadband/IPTV sekitar 2,6 juta. Perseroan bersama Entitas Anak memperoleh pangsa pasar dengan menerapkan strategi multi brand (Indovision, OkeVision dan Top TV), dimana pada Desember 2017 menjadi kesatuan dalam brand MNC Vision dan memiliki brand awareness yang kuat. Sebagai market leader MNC Vision, MNC Play dan MNC Now diuntungkan dengan memiliki jumlah pelanggan yang besar sehingga memudahkan negosiasi harga berlangganan dengan content provider, harga pembelian peralatan dengan supplier fixed asset, dan negosiasi komisi dengan dealer pihak ketiga. Entitas Anak Memiliki Teknologi dan Infrastruktur Terbaik untuk Memberikan Servis Unggul kepada Para Pelanggan. Dalam memberikan pelayanan kepada pelanggan, Entitas Anak (MNC Vision, MNC Play dan MNC Now) memiliki dan mengoperasikan infrastruktur dengan jaringan distribusi yang luas, termasuk Fiber Optic, jaringan DTH dan satelit. Infrastruktur yang dimiliki oleh Entitas Anak (MNC Vision, MNC Play dan MNC Now) memegang peranan penting dalam kualitas servis yang diberikan kepada pelanggan. Semakin lengkap dan canggih teknologi infrastruktur, maka Entitas Anak (MNC Vision, MNC Play dan MNC Now) dapat memberikan pelayanan prima sepanjang waktu tanpa gangguan cuaca maupun signal. Dari sisi cakupan jaringan, Perseroan melalui Entitas Anaknya juga memiliki jaringan fiber super highway yang membentang hingga mencapai 1.165 km di sepanjang Pulau Jawa, dari Jakarta hingga Surabaya. -

DFA Canada World Equity Portfolio - Class F As of March 31, 2021 (Updated Monthly) Source: RBC Holdings Are Subject to Change
DFA Canada World Equity Portfolio - Class F As of March 31, 2021 (Updated Monthly) Source: RBC Holdings are subject to change. The information below represents the portfolio's holdings (excluding cash and cash equivalents) as of the date indicated, and may not be representative of the current or future investments of the portfolio. The information below should not be relied upon by the reader as research or investment advice regarding any security. This listing of portfolio holdings is for informational purposes only and should not be deemed a recommendation to buy the securities. The holdings information below does not constitute an offer to sell or a solicitation of an offer to buy any security. The holdings information has not been audited. By viewing this listing of portfolio holdings, you are agreeing to not redistribute the information and to not misuse this information to the detriment of portfolio shareholders. Misuse of this information includes, but is not limited to, (i) purchasing or selling any securities listed in the portfolio holdings solely in reliance upon this information; (ii) trading against any of the portfolios or (iii) knowingly engaging in any trading practices that are damaging to Dimensional or one of the portfolios. Investors should consider the portfolio's investment objectives, risks, and charges and expenses, which are contained in the Prospectus. Investors should read it carefully before investing. This fund operates as a fund-of-funds and generally allocates its assets among other mutual funds, but has the ability to invest in securities and derivatives directly. The holdings listed below contain both the investment holdings of the corresponding underlying funds as well as any direct investments of the fund. -
Phase 3 Dissemination Additions
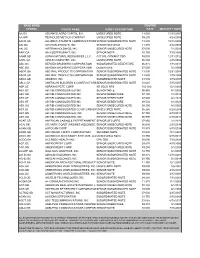
NASD BOND COUPON SYMBOL ISSUER NAME SHORT DESCRIPTION RATE MATURITY DATE AA.GA ADVANCE AGRO CAPITAL B.V. UNSECURED NOTE 13.000 11/15/2007 AA.GW REYNOLDS METALS COMPANY UNSECURED NOTE 09.200 4/24/2006 AACB.GA ALLIANCE ATLANTIS COMMUNICATIONSSENIOR SUBORDINATED NOTE 13.000 12/15/2009 AAI.GA AIRTRAN AIRWAYS, INC. SENIOR SECURED 11.270 4/12/2008 AAI.GC AIRTRAN HOLDINGS, INC. SENIOR UNSECURED NOTE 07.000 7/1/2023 AAIF.GA AAI.FOSTERGRANT, INC. SENIOR NOTE 10.750 7/15/2006 AANR.GB ALPHA NATURAL RESOURCES L.L.C. ACTUAL CPN/MAT TBD 00.000 12/31/2012 AAPL.GA APPLE COMPUTER, INC. UNSECURED NOTE 06.500 2/15/2004 ABC.GC BERGEN BRUNSWIG CORPORATION SUBORDINATED DEBENTURE 06.875 7/15/2011 ABC.GD BERGEN BRUNSWIG CORPORATION DEBENTURE 07.000 3/1/2006 ABCR.GA ABC RAIL PRODUCTS CORPORATION SENIOR SUBORDINATED NOTE 11.500 12/31/2004 ABCR.GB ABC RAIL PRODUCTS CORPORATION SENIOR SUBORDINATED NOTE 11.500 1/15/2004 ABGX.GB ABGENIX, INC SUBORDINATED NOTE 03.500 3/15/2007 ABLC.GA AMERICAN BUILDERS & CONTRACTORSSENIOR SUBORDINATED NOTE 10.625 5/15/2007 ABP.GE ABRAXAS PETE CORP. SR SECD NTS 100.000 12/1/2009 ABY.GC ABITIBI-CONSOLIDATED INC. SENIOR NOTE 06.950 4/1/2008 ABY.GD ABITIBI-CONSOLIDATED INC. SENIOR DEBENTURE 07.400 4/1/2018 ABY.GE ABITIBI-CONSOLIDATED INC. SENIOR DEBENTURE 07.500 4/1/2028 ABY.GF ABITIBI-CONSOLIDATED INC. SENIOR DEBENTURE 08.500 8/1/2029 ABY.GG ABITIBI-CONSOLIDATED INC SENIOR UNSECURED NOTE 08.300 8/1/2005 ABY.GJ ABITIBI-CONSOLIDATED CO OF CANADAUNSECURED NOTE 05.250 6/20/2008 ABY.GN ABITIBI-CONSOLIDATED, INC. -

Download Line up Channel MNC Vision on Vision+
LINE UP CHANNEL Berlaku mulai Juni 2020 VISION+ FREE (46) NO. GENRE CHANNELS CATCH UP 1 RCTI YES 2 GTV YES 3 MNCTV YES 4 iNEWS YES 5 SCTV YES 6 INDOSIAR YES 7 METROTV YES 8 TRANS7 YES 9 ANTV YES 10 TVRI YES 11 FTA LOCAL NET YES 12 KOMPAS TV YES 13 RTV YES 14 BERITA SATU YES 15 JAKTV YES 16 BALI TV YES 17 JTV YES 18 DAAITV YES 19 TV9 NUSANTARA YES 20 TAWAF TV YES 21 TVMU YES 22 VISION PRIME YES 23 MNC SPORT YES 24 IDX CHANNEL YES 25 ENTERTAINMENT YES 26 MNC NEWS YES 27 MNC SHOP SMART YES 28 MNC CHANNELS MNC SHOP TRENDY YES 29 MUSIK TV YES 30 INFOTAINMENT YES 31 LIFE YES 32 LIFESTYLE & FASHION YES 33 MUSLIM TV YES 34 OK TV YES 35 AL JAZEERA ENGLISH YES 36 AL QURAN AL KAREEM YES 37 ANHUI TV YES 38 CLUBBING TV YES 39 EWTN YES 40 FRANCE 24 YES FTA INTERNATIONAL 41 HUNAN TV YES 42 JIANGSU TV YES FTA INTERNATIONAL 43 RT ENGLISH YES 44 TRT WORLD YES 45 SHANGHAI DRAGON TV YES 46 XING KONG TV YES VISION+ PAY (59) NO. GENRE CHANNELS CATCH UP 1 RCTI YES 2 GTV YES 3 MNCTV YES 4 iNEWS YES 5 SCTV YES 6 INDOSIAR YES 7 METROTV YES 8 TRANS7 YES 9 ANTV YES 10 TVRI YES 11 FTA LOCAL NET YES 12 KOMPAS TV YES 13 RTV YES 14 BERITA SATU YES 15 JAKTV YES 16 BALI TV YES 17 JTV YES 18 DAAITV YES 19 TV9 NUSANTARA YES 20 TAWAF TV YES 21 TVMU YES 22 VISION PRIME YES 23 MNC SPORT YES 24 MNC SPORTS 2 YES 25 IDX CHANNEL YES 26 ENTERTAINMENT YES 27 MNC NEWS YES 28 MNC SHOP SMART YES 29 MNC SHOP TRENDY YES MNC CHANNELS 30 MUSIC TV YES 31 INFOTAINMENT YES 32 LIFE YES 33 LIFESTYLE & FASHION YES 34 MUSLIM TV YES 35 OK TV YES 36 BESMART YES 37 KIDS TV YES 38 AL JAZEERA ENGLISH YES 39 AL QURAN AL KAREEM YES 40 ANHUI TV YES 41 CLUBBING TV YES 42 EWTN YES FTA INTERNATIONAL 43 FRANCE 24 YES 44 FTA INTERNATIONAL HUNAN TV YES 45 JIANGSU TV YES 46 RT ENGLISH YES 47 TRT WORLD YES 48 SHANGHAI DRAGON TV YES 49 XING KONG TV YES 50 DW YES 51 BOOMERANG YES 52 HITS YES 53 HITS MOVIES YES 54 THRILL YES 55 PREMIUM CHANNELS TVN YES 56 TVN MOVIES YES 57 ZEE BIOSKOP YES 58 CELESTIAL CLASSIC MOVIES YES 59 KIX YES MNC VISION connect (98+14) NO. -

T O W Ards a New Vision
PT MNC Sky Vision Tbk TOWARDS A TOWARDS NEW VISION NEW 2017 Laporan Tahunan Annual Report Laporan Tahunan Laporan Tahunan MNC VISION TOWER Jl. Raya Panjang Blok Z / III 2017 Green Garden, Jakarta 11520 Indonesia Annual Report Hotline : 1500 900 Phone : +6221 582 8000 Ext. 370101 Laporan Tahunan 2017 Annual Report Fax : +6221 391 4600 PT MNC Sky Vision Tbk Website : www.mncvision.id MEMPERKENALKAN Introducing Pada tahun 2017, kami mengawali perjalanan dengan In 2017, we started the journey with a new identity as identitas baru sebagai MNC Vision. Evolusi ini menjadi MNC Vision. This evolution became a manifestation of our perwujudan komitmen kami dalam menyediakan tayangan commitment in providing quality shows in line with the bermutu, sesuai visi Perseroan sebagai pilihan pertama Company’s vision, as the first choice of Pay TV subscribers pelanggan TV berlangganan di Indonesia. in Indonesia. DAFTAR ISI Table of Contents Kinerja 2017 4 1 2017 Performance Laporan Manajemen 10 2 Management Report Informasi Umum 44 3 General Information Analisis dan Pembahasan Manajemen 74 4 Management’s Discussion and Analysis Tata Kelola Perusahaan 104 5 Corporate Governance Tanggung Jawab Sosial Perusahaan 210 6 Corporate Social Responsibility Laporan Keuangan 242 7 Financial Report PENJELASAN TEMA Theme Description PT MNC Sky Vision Tbk (MSKY) sebagai pionir TV PT MNC Sky Vision Tbk (MSKY) as a pioneer of Pay TV berlangganan di Indonesia telah membuktikan in Indonesia has proven its presence for over 23 years eksistensinya selama lebih dari 23 tahun dalam in providing the best service for the Indonesian people. menyediakan layanan terbaik bagi masyarakat Indonesia. In 2017, the Pay TV industry became more dynamic Di tahun 2017, kondisi industri TV berlangganan semakin with the increasing competition and innovation. -

MNC Bank and Atome Financial Sign Strategic Partnership to Personalize Digital Financial Services
MNC Bank and Atome Financial Sign Strategic Partnership to Personalize Digital Financial Services JAKARTA, 29 June 2021 — PT MNC Kapital Indonesia Tbk (“BCAP”), through its subsidiaries, PT MNC Bank Internasional Tbk (“BABP”) and PT MNC Teknologi Nusantara have signed a strategic partnership with Atome Financial to expand the range of tailored digital financial services to Indonesian consumers. The partnership with Atome Financial and its affiliates covers digital loan channelling with Kredit Pintar, Atome’s buy now pay later product, AI-based credit scoring, and underwriting to lend safely and re- sponsibly. Besides increasing MotionBanking, Kredit Pintar and Atome’s user bases, the collaboration will offer extra convenience via simple and easy digital banking services. The partnership’s highlights include: MotionBanking and Kredit Pintar will offer digital loans Borrowers can apply for the loan directly from MotionBanking or Kredit Pintar app using the QR code shown on MNC Group's me- dia platforms. Fast loan approval from Kredit Pintar along with MotionBanking's complete digital banking services will create a convenient user journey, especially with auto-debit feature for monthly repayment. MotionPay to offer cash loans through Kredit Pintar and option to pay with Atome buy now pay later Users can apply directly through MotionPay app to get a cash loan and/or BNPL payment option. Users can get fast and secure cash loan tailored to their needs. Users can also use the buy now pay later payment option for both online and offline transactions. This synergy is the first in Indonesia, where Buy Now Pay Later As previously announced, transaction is executed through MotionPay with funding from MNC Bank recently launched MotionBanking channeled through Kredit Pintar and Atome. -

Albuquerque Morning Journal, 07-19-1910 Journal Publishing Company
University of New Mexico UNM Digital Repository Albuquerque Morning Journal 1908-1921 New Mexico Historical Newspapers 7-19-1910 Albuquerque Morning Journal, 07-19-1910 Journal Publishing Company Follow this and additional works at: https://digitalrepository.unm.edu/abq_mj_news Recommended Citation Journal Publishing Company. "Albuquerque Morning Journal, 07-19-1910." (1910). https://digitalrepository.unm.edu/ abq_mj_news/4077 This Newspaper is brought to you for free and open access by the New Mexico Historical Newspapers at UNM Digital Repository. It has been accepted for inclusion in Albuquerque Morning Journal 1908-1921 by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. A.LBUQUEEQUE MOBNING JOURNAL. Blngin Coplea. 1 TRTY-SEC0N- D YEAR. Vol. CXXVII., No. 19. ALBUQUERQUE, NEW MEXICO, TUESDAY, JULY 19, 1910, By Wail, lOcta. Month f crnU, 11 y Carrier, 60 eeula a Month. "It was explained that If there wns court on appptl. The award of the any question of Indefiniteness as to commission, to which exception Ik ON the date when the standardisation was FEARFOL TOLL taken, was $200.000, The appeal will SPEAKER JOKE 10,000 MEN to take effect, we would agree that ll not interfere with progress of reclu WILL SO BACK TO CONGRESS should be not later than January 1. motion work at the dam site as the 1913, or earlier, If the board of rail- government has deposited the amount way upon hearing commissioners, of of the award with the Socorro dis- the facts in the case, should so deter- trict clerk. mine. SAYS 10 KANSAS GRAND TRUNK "This was not considered satisfac- OF LIVES IN IF 600 SPARES LIFE tory and the conference was adjourned BUTTE CHIEF OF POLICE until the afternoon, when we were LET OUT PENDING INQUIRY handed the schedule of rates of pay and rules which are practically the eastern standard. -

MNC Vision Networks (IPTV) IPTV Takeaways: Undertaking a Synergistic Model
Equity Research IPTV Company Update Monday,28 October 2019 Not Rated MNC Vision Networks (IPTV) IPTV Takeaways: Undertaking a synergistic model Last price (IDR) 500 We followed the MVN team recently to find out more about its OTT platform, the potential acquisition of its internet peer, and the potential sale-lease back of its home passes (HPs). MVN is a growth stock whose underlying strategy is to consolidate its tech platforms and offer a unique content proposition. MVN is endowed with original content. MNC Now is the “Netflix” lever for Stock Statistics MVN selling OTT content to build stickiness. MNC Now is also envisaged to get mobile users onto the MVN content platform. This OTT is adapted for Sector Telco – Media short/medium original content and MNC libraries to generate advertising Bloomberg Ticker IPTV IJ revenues first, with a view to charge subscription fees later. No other internet No of Shrs (mn) 31,702 player has access to that content and not many players are producing either. The MAU is at 10mn and MVN expects strong growth to 60mn by YE2020. Mkt. Cap (IDRbn/USDmn) 16,742/1,209 Avg. daily T/O (IDRbn/USDmn) 17.0/1.1 MVN is keen to buy out its competitor. MNC group has shown a strong commitment to scale up MVN even after failed attempts to partner with Major shareholders (%) Vivendi. We attended MVN’s 9/10/19 presentation where it stated its plan to Global Mediacom 71.5 acquire a fixed internet peer so that MVN could potentially own 4mn home passes if the transaction could be concluded (~1.5mn MVN standalone). -

PT MNC Vision Networks Tbk Paparan Publik Jakarta, 28 Juli 2019 the Most Integrated Subscription Platform with Content Superiority
Listed and traded on the Indonesia Stock Exchange STOCK CODE: IPTV PT MNC Vision Networks Tbk Paparan Publik Jakarta, 28 Juli 2019 The Most Integrated Subscription Platform with Content Superiority * As of March 2020 PUBLIC PT MNC Vision Networks Tbk (IPTV) 73.32% 26.58% 91.90% 80% 99.99% 100% 99.90% 99% 100% PT MNC Sky Vision Tbk PT Digital Vision Nusantara PT MNC Kabel Mediakom Play Box PT MNC OTT Network PT Mitra Operator Local MNC Channels LCO DTH post-paid Pay TV DTH pre-paid Pay TV Broadband & IPTV Android TV OTT Box OTT – TV Anywhere Local Cable Operator Pay Channel & VOD • Broadening reach of • Produces and • DTH KU-Band for • TV anywhere for MVN to untapped • Over 90% market • Provides high speed • Distribution through operates 12 pay cost efficient MVN subscribers. Pay-TV Market all share with 2.1m FTTH broadband and MNC Vision, MNC channels. services, capturing over Indonesia. subscribers. interactive IPTV Play, digital channel • Vision+ has over 29 mass market • Produces original services. (e-commerce), and mio MAU. • LCO Partnership • DTH S-band (2.520 – segment. content suited for retail dealership. through 2.670 Ghz) for • 3rd largest • Live TV, catch up TV, digital platform • Acquires 8,000 - digitalization or extensive nationwide broadband with 1.5 • Selling Play Box library, VOD, and (short and medium 9,000 new content licensing & coverage. mio home pass and using 3rd party original content, form content). subscribers on a acquisition close to 300,000 broadband network. both short and mid • Hidden treasure: 5G daily basis. • MNC Channels has subs.