Peach Fruit Quality Analysis in Relation to Organic and Conventional Cultivation Techniques
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
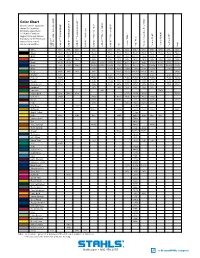
Color Chart ® ® ® ® Closest Pantone® Equivalent Shown
™ ™ II ® Color Chart ® ® ® ® Closest Pantone® equivalent shown. Due to printing limitations, colors shown 5807 Reflective ® ® ™ ® ® and Pantone numbers ® ™ suggested may vary from ac- ECONOPRINT GORILLA GRIP Fashion-REFLECT Reflective Thermo-FILM Thermo-FLOCK Thermo-GRIP ® ® ® ® ® ® ® tual colors. For the truest color ® representation, request Scotchlite our material swatches. ™ CAD-CUT 3M CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT Felt Perma-TWILL Poly-TWILL Thermo-FILM Thermo-FLOCK Thermo-GRIP Vinyl Pressure Sensitive Poly-TWILL Sensitive Pressure CAD-CUT White White White White White White White White White* White White White White White Black Black Black Black Black Black Black Black Black* Black Black Black Black Black Gold 1235C 136C 137C 137C 123U 715C 1375C* 715C 137C 137C 116U Red 200C 200C 703C 186C 186C 201C 201C 201C* 201C 186C 186C 186C 200C Royal 295M 294M 7686C 2747C 7686C 280C 294C 294C* 294C 7686C 2758C 7686C 654C Navy 296C 2965C 7546C 5395M 5255C 5395M 276C 532C 532C* 532C 5395M 5255C 5395M 5395C Cool Gray Warm Gray Gray 7U 7539C 7539C 415U 7538C 7538C* 7538C 7539C 7539C 2C Kelly 3415C 341C 340C 349C 7733C 7733C 7733C* 7733C 349C 3415C Orange 179C 1595U 172C 172C 7597C 7597C 7597C* 7597C 172C 172C 173C Maroon 7645C 7645C 7645C Black 5C 7645C 7645C* 7645C 7645C 7645C 7449C Purple 2766C 7671C 7671C 669C 7680C 7680C* 7680C 7671C 7671C 2758U Dark Green 553C 553C 553C 447C 567C 567C* 567C 553C 553C 553C Cardinal 201C 188C 195C 195C* 195C 201C Emerald 348 7727C Vegas Gold 616C 7502U 872C 4515C 4515C 4515C 7553U Columbia 7682C 7682C 7459U 7462U 7462U* 7462U 7682C Brown Black 4C 4675C 412C 412C Black 4C 412U Pink 203C 5025C 5025C 5025C 203C Mid Blue 2747U 2945U Old Gold 1395C 7511C 7557C 7557C 1395C 126C Bright Yellow P 4-8C Maize 109C 130C 115U 7408C 7406C* 7406C 115U 137C Canyon Gold 7569C Tan 465U Texas Orange 7586C 7586C 7586C Tenn. -

RAL COLOR CHART ***** This Chart Is to Be Used As a Guide Only. Colors May Appear Slightly Different ***** Green Beige Purple V
RAL COLOR CHART ***** This Chart is to be used as a guide only. Colors May Appear Slightly Different ***** RAL 1000 Green Beige RAL 4007 Purple Violet RAL 7008 Khaki Grey RAL 4008 RAL 7009 RAL 1001 Beige Signal Violet Green Grey Tarpaulin RAL 1002 Sand Yellow RAL 4009 Pastel Violet RAL 7010 Grey RAL 1003 Signal Yellow RAL 5000 Violet Blue RAL 7011 Iron Grey RAL 1004 Golden Yellow RAL 5001 Green Blue RAL 7012 Basalt Grey Ultramarine RAL 1005 Honey Yellow RAL 5002 RAL 7013 Brown Grey Blue RAL 1006 Maize Yellow RAL 5003 Saphire Blue RAL 7015 Slate Grey Anthracite RAL 1007 Chrome Yellow RAL 5004 Black Blue RAL 7016 Grey RAL 1011 Brown Beige RAL 5005 Signal Blue RAL 7021 Black Grey RAL 1012 Lemon Yellow RAL 5007 Brillant Blue RAL 7022 Umbra Grey Concrete RAL 1013 Oyster White RAL 5008 Grey Blue RAL 7023 Grey Graphite RAL 1014 Ivory RAL 5009 Azure Blue RAL 7024 Grey Granite RAL 1015 Light Ivory RAL 5010 Gentian Blue RAL 7026 Grey RAL 1016 Sulfer Yellow RAL 5011 Steel Blue RAL 7030 Stone Grey RAL 1017 Saffron Yellow RAL 5012 Light Blue RAL 7031 Blue Grey RAL 1018 Zinc Yellow RAL 5013 Cobolt Blue RAL 7032 Pebble Grey Cement RAL 1019 Grey Beige RAL 5014 Pigieon Blue RAL 7033 Grey RAL 1020 Olive Yellow RAL 5015 Sky Blue RAL 7034 Yellow Grey RAL 1021 Rape Yellow RAL 5017 Traffic Blue RAL 7035 Light Grey Platinum RAL 1023 Traffic Yellow RAL 5018 Turquiose Blue RAL 7036 Grey RAL 1024 Ochre Yellow RAL 5019 Capri Blue RAL 7037 Dusty Grey RAL 1027 Curry RAL 5020 Ocean Blue RAL 7038 Agate Grey RAL 1028 Melon Yellow RAL 5021 Water Blue RAL 7039 Quartz Grey -

Chapter 1 Definitions and Classifications for Fruit and Vegetables
Chapter 1 Definitions and classifications for fruit and vegetables In the broadest sense, the botani- Botanical and culinary cal term vegetable refers to any plant, definitions edible or not, including trees, bushes, vines and vascular plants, and Botanical definitions distinguishes plant material from ani- Broadly, the botanical term fruit refers mal material and from inorganic to the mature ovary of a plant, matter. There are two slightly different including its seeds, covering and botanical definitions for the term any closely connected tissue, without vegetable as it relates to food. any consideration of whether these According to one, a vegetable is a are edible. As related to food, the plant cultivated for its edible part(s); IT botanical term fruit refers to the edible M according to the other, a vegetable is part of a plant that consists of the the edible part(s) of a plant, such as seeds and surrounding tissues. This the stems and stalk (celery), root includes fleshy fruits (such as blue- (carrot), tuber (potato), bulb (onion), berries, cantaloupe, poach, pumpkin, leaves (spinach, lettuce), flower (globe tomato) and dry fruits, where the artichoke), fruit (apple, cucumber, ripened ovary wall becomes papery, pumpkin, strawberries, tomato) or leathery, or woody as with cereal seeds (beans, peas). The latter grains, pulses (mature beans and definition includes fruits as a subset of peas) and nuts. vegetables. Definition of fruit and vegetables applicable in epidemiological studies, Fruit and vegetables Edible plant foods excluding -

Green Beige Beige Sand Yellow Signal Yellow Golden Yellow Honey Yellow Maize Yellow Daffodil Yellow Brown Beige Lemon Yellow
GREEN BEIGE BEIGE SAND YELLOW SIGNAL YELLOW GOLDEN YELLOW DAFFODIL HONEY YELLOW MAIZE YELLOW YELLOW BROWN BEIGE LEMON YELLOW OYSTER WHITE IVORY LIGHT IVORY SULFUR YELLOW SAFFRON YELLOW ZINC YELLOW GREY BEIGE OLIVE YELLOW COLZA YELLOW TRAFFIC YELLOW LUMINOUS OCHRE YELLOW YELLOW CURRY MELON YELLOW BROOM YELLOW DAHLIA YELLOW PASTEL YELLOW PEARL BEIGE PEARL GOLD SUN YELLOW YELLOW ORANGE RED ORANGE VERMILION PASTEL ORANGE PURE ORANGE LUMINOUS LUMINOUS BRIGHT RED ORANGE BRIGHT ORANGE ORANGE TRAFFIC ORANGE SIGNAL ORANGE DEEP ORANGE SALMON ORANGE PEARL ORANGE FLAME RED SIGNAL RED CARMINE RED RUBY RED PURPLE RED WINE RED BLACK RED OXIDE RED BROWN RED BEIGE RED TOMATO RED ANTIQUE PINK LIGHT PINK CORAL RED ROSE LUMINOUS STRAWBERRY RED TRAFFIC RED SALMON PINK LUMINOUS RED BRIGHT RED RASPBERRY RED PURE RED ORIENT RED PEARL RUBY RED PEARL PINK RED LILAC RED VIOLET HEATHER VIOLET CLARET VIOLET BLUE LILAC TRAFFIC PURPLE PURPLE VIOLET SIGNAL VIOLET PASTEL VIOLET TELEMAGENTA PEARL BLACK PEARL VIOLET BERRY ULTRAMARINE VIOLET BLUE GREEN BLUE BLUE SAPHIRE BLUE BLACK BLUE SIGNAL BLUE BRILLANT BLUE GREY BLUE AZURE BLUE GENTIAN BLUE STEEL BLUE LIGHT BLUE COBALT BLUE PIGEON BLUE SKY BLUE TRAFFIC BLUE TURQUOISE BLUE CAPRI BLUE OCEAN BLUE WATER BLUE PEARL GENTIAN PEARL NIGHT NIGHT BLUE DISTANT BLUE PASTEL BLUE BLUE BLUE PATINA GREEN EMERALD GREEN LEAF GREEN OLIVE GREEN BLUE GREEN MOSS GREEN GREY OLIVE BOTTLE GREEN BROWN GREEN FIR GREEN GRASS GREEN RESEDA GREEN BLACK GREEN REED GREEN YELLOW OLIVE TURQUOISE BLACK OLIVE GREEN MAY GREEN YELLOW GREEN PASTEL GREEN CHROME -

Maize Variety Choices and Mayan Foodways in Rural Yucatan, Mexico
All Maize Is Not Equal: Maize Variety Choices and Mayan Foodways in Rural Yucatan, Mexico John Tuxill, Luis Arias Reyes, Luis Latournerie Moreno, and Vidal Cob Uicab, Devra I. Jarvis Introduction Foodways have changed substantially over the past several centuries in the Yucatan Peninsula and other areas of Mesoamerica, but one constant is the presence of maize (Zea mays) at the heart of rural economy, ecology, and culture. The domes- tication and diversification of maize – the world’s most productive grain crop – by indigenous farmers ranks as one of the greatest accomplishments of plant breeding. Remains of ancient maize cobs in the archeological record suggest that maize was first brought into cultivation roughly 7,000 years ago in the highlands of central Mexico, where its closest wild relative, teosinte (Zea mays ssp. parviglumis), also grows (Wilkes 1977; McClung de Tapia 1997; Smith 2001; Piperno and Flannery 2001; Matsuoka et al. 2002). From that starting point, maize was gradually selected and diversified over time by farmers into an impressive array of different forms, sizes, and colors. Maize appears to spread out of central Mexico rapidly in the context of regional trade and exchange networks, and farmers selected and adapted maize populations to thrive in new environments. Archeobotanical evidence from northern Belize suggests maize arrived in the Yucatan Peninsula by about 5,000 years B.P. (Colunga-García Marín and Zizumbo-Villarreal 2004). As maize spread and evolved in the Yucatan at the hands of Mayan farmers, it achieved a symbolic, ceremonial, ecological, and economic importance surpassing that of any other plant or natural resource in the Mayan world. -

NARANI RED OCHRE MINING PROJECT (M.L No
NARANI RED OCHRE MINING PROJECT (M.L No. 44/2006, ML Area: 259.0 ha.) Located at near Village: Narani, Tehsil: Choti Sadari, District: Pratapgarh (Raj.) Pre Feasibility Report PRE-FEASIBILITY REPORT (As per Ministry of Environment and Forests Letter dated: 30-12-2010) 1. EXECUTIVE SUMMARY Particulars Details Project name Proposed expansion project of Narani Red Ochre Mine Location Near Village: Narani, Tehsil: Choti Sadri & District- Pratapgarh, Rajasthan (M.L. No.: 44/2006) GPS Co-Ordinates of S. Reference Latitude Longitude Project No Point No. FRP 24°24'47.39” 74°46'43.95” 1 A 24°24'54.57” 74°47'2.8” 2 B 24°25'16.47” 74°46'27.23” 3 C 24°26'14.37” 74°47'9.74” 4 D 24°25'52.47” 74°47'45.32” Toposheet No. Falls in survey of India Toposheet No.- 45L/15 Total Mine area 259.0 Hectare (Govt. waste land: 60.2966 ha., Pvt. Land: 165.2862 ha., Grazing land: 33.4172 ha.) Total Reserves 4,29,38,953 MT (Mineable Reserves: 3,64,98,112 MT) Capacity/year Proposed production – 5,88,236 MT (ROM) Life of Mine ~73 years Estimated project cost 2 Crore/- EMP Cost 4.40 Lac/- per annum Power Requirement Electric connection is already taken for office purpose. Fuel Requirement As per requirement, will be made available by contractor Alternate source of power is DG Set of 63 KVA available in the DG Set mining lease area for office purpose. Highest and Lowest S. No. Particulars Elevation (mRL) Elevation 1. -

Air Force Blue (Raf) {\Color{Airforceblueraf}\#5D8aa8
Air Force Blue (Raf) {\color{airforceblueraf}\#5d8aa8} #5d8aa8 Air Force Blue (Usaf) {\color{airforceblueusaf}\#00308f} #00308f Air Superiority Blue {\color{airsuperiorityblue}\#72a0c1} #72a0c1 Alabama Crimson {\color{alabamacrimson}\#a32638} #a32638 Alice Blue {\color{aliceblue}\#f0f8ff} #f0f8ff Alizarin Crimson {\color{alizarincrimson}\#e32636} #e32636 Alloy Orange {\color{alloyorange}\#c46210} #c46210 Almond {\color{almond}\#efdecd} #efdecd Amaranth {\color{amaranth}\#e52b50} #e52b50 Amber {\color{amber}\#ffbf00} #ffbf00 Amber (Sae/Ece) {\color{ambersaeece}\#ff7e00} #ff7e00 American Rose {\color{americanrose}\#ff033e} #ff033e Amethyst {\color{amethyst}\#9966cc} #9966cc Android Green {\color{androidgreen}\#a4c639} #a4c639 Anti-Flash White {\color{antiflashwhite}\#f2f3f4} #f2f3f4 Antique Brass {\color{antiquebrass}\#cd9575} #cd9575 Antique Fuchsia {\color{antiquefuchsia}\#915c83} #915c83 Antique Ruby {\color{antiqueruby}\#841b2d} #841b2d Antique White {\color{antiquewhite}\#faebd7} #faebd7 Ao (English) {\color{aoenglish}\#008000} #008000 Apple Green {\color{applegreen}\#8db600} #8db600 Apricot {\color{apricot}\#fbceb1} #fbceb1 Aqua {\color{aqua}\#00ffff} #00ffff Aquamarine {\color{aquamarine}\#7fffd4} #7fffd4 Army Green {\color{armygreen}\#4b5320} #4b5320 Arsenic {\color{arsenic}\#3b444b} #3b444b Arylide Yellow {\color{arylideyellow}\#e9d66b} #e9d66b Ash Grey {\color{ashgrey}\#b2beb5} #b2beb5 Asparagus {\color{asparagus}\#87a96b} #87a96b Atomic Tangerine {\color{atomictangerine}\#ff9966} #ff9966 Auburn {\color{auburn}\#a52a2a} #a52a2a Aureolin -

Maxon; Lake Cha.Mplain, 1901, Eggleston
THE NORTH AMERICAN SPECIES OF NYMPHA EA. By GERRIT S. MILLER, Jr., and PAUL C. STANDLEY. INTRODUCTION. NOTE BY ;T. N. ROSE. There are some groups of plants whoso taxonomy can bo fairly well understood almost solely from ordinary herbarium material. There are others in which it is impossible to understand the true relationships from such material alone, anrl some evon in which herbarium specimens are almost useless. Among the last are the Cactaceae, many of the tropical Euphorbias, the Crassulaceae, and in general all the succulent plants. To be properly understood such plants should be soon growing, or should be studied from material preserved in alcohol or formalin, since in dry specimens most of the important characters are distorted beyond all possibility of recogni tion. Among plants of this kind the Nymphaeaceae must be included. Most of our knowledge of the genus Nymphaea, judging from the literature of the North American species at least, has heen derived from the study of dried herbarium material. As a result, different authors have arrived at very different conclusions. Impor tant peculiarities of habit Ilnd strueture not discernible in such specimens have been overlooked, and our knowledge of the genus has not kept pace with the advances made in some other groups. About ten years ago Mr. Gcrrit S. Miller, jr., became interested in the genus Nymphaea from field observations of the plants occurring in central New York and in the vicinity of Washington, currently regarded as belonging to one species. Study of fresh and forlllalin preserved material showed that there were important and easily recognizable differences between the northern form and that found farther south, differences in habit, color, and structure, scarcely to be detected in dried specimens. -

An Overview of Vertebrate Pests in India
UC Agriculture & Natural Resources Proceedings of the Vertebrate Pest Conference Title An Overview of Vertebrate Pests in India Permalink https://escholarship.org/uc/item/7hw2r9p9 Journal Proceedings of the Vertebrate Pest Conference, 22(22) ISSN 0507-6773 Author Sridhara, Shakunthala Publication Date 2006 DOI 10.5070/V422110078 eScholarship.org Powered by the California Digital Library University of California An Overview of Vertebrate Pests in India ShakunthalaSridhara AINPonRodentControl,CollegeofAgriculture,UniversityofAgriculturalSciences,Bangalore,India ABSTRACT : Abillion-plushumanpopulation,agriculture,anddevelopmentareshrinkinganddegradingthehabitatofmanyof the1,200birdand500mammalspeciesofIndia.Withhumansandherbivorescompetingforthesameresources,manyofthemare becomingpestsoncrops.Thegranivorousbirdsdepredateonselectedcereals,sunflower,groundnut,andoilpalm.Guava,grape, apple,sapota,pecan,pomegranate,andpineapplearedamagedsignificantlybyfrugivorebirds.Amongstvertebratepests,rodents arethemostdestructive.Adozenspecies,viz. Rattusrattus ,Bandicotabengalensis ,B.indica ,Millardiameltada ,Musbooduga , M.platythrix ,Musmusculus ,Tateraindica ,Merioneshurrianae ,Funambuluspennanti ,F.palmarum ,F .tristriatus ,and Hystrix indica areseriouspests.Cereals,pulses,oilseeds,vegetables,fruits,andplantationcropsaredamagedconsiderably.Sownseeds, seedlingsofmaize,sorghum,sunflower,groundnut,redgram,tendercoconut,oilpalm,cardamom,andcocoaaredepredatedmuch more.Othervertebratepestsofsignificanceare Pteropusgiganteus ,Cynopterussphinx ,Rousettusleschenaulti(bats),Boselaphus -

Races of Maize in Ecuador
RACES OF MAIZE IN ECUADOR David H. Timothy William H. Hatheway Ulysses J. Grant Manuel Torregroza C. Daniel Sarria V. Daniel Varela A. NATIONAL ACADEMY OF SCIENCES- NATIONAL RESEARCH COUNCIL Publication 975 COMMITTEE ON PRESERVATION OF indigenous STRAINS OF MAIZE OF THE AGRICULTURALBOARD DIVISIONOF BIOLOGYAND AGRICULTURE NATIONALACADEMY OF SCIENCES-NATIONALRESEARCH COUNCLL Ralph E. Cleland, Chairman Edgar Anderson Claud L. Horn Paul C. Mangelsdorf William L. Brown Merle T. Jenkins G. H. Stringfield C. O. Erlanson George F. Sprague Funds were provided for this publication by a contract between the National Academy of Sciences-National Research Council and The Institute of Inter-American Affairs of the International Cooperation Administration. The grant was made for the work of the Committee on Preservation of Indigenous Strains of Maize, under the Agricul- tural Board. The AGRICULTURAL BOARD, a part of the Division of Biology and Agriculture of the National Academy of Sciences-National Re- search Council, studies and reports on scientific problems of agri- culture in relation to the national economy. Financial support for the meetings and publications of the Board is derived primarily from the Agricultural Research Institute, which is an organization com- posed of representatives of industry, trade organizations, academic institutions, and governmental agencies concerned with agriculture. Members of the A agricultural Board and of its committees serve with- out compensation beyond their actual expenses. Funds for the work of the Agricultural Board are received and administered by the Academy. RACES OF MAIZE IN ECUADOR RACES OF MAIZE IN ECUADOR David H. Timothy, William H. Hatheway, Ulysses J. Grant, Manuel Torregroza C.,Daniel Sarria V., Daniel Varela A. -

July 31, 1956 R
July 31, 1956 R. L. BYRUM Plant Pat. 1,504 ROSE PLANT Filed Oct. 10, 1955 Plant Pat. 1,504 United States Patent 0 "PICC Patented July 31, 1956 2 FLOWER Locality where grown and observed: Richmond, Indiana. 1,504 Flowers borne: l to stem; on strong stems of medium ROSE PLANT length. Quantity of bloom: Abundant, in greenhouse. Roy L. Byrum, Richmond, Ind., assignor to Joseph H. Continuity: Continuous. Hill Company, Richmond, Ind., a corporation of In Fragrance: Slight. Nature—tea. diana Bud: Application October 10, 1955, Serial No. 539,708 10 Peduncle.-Medium length; medium diameter; strong; erect. Bark--sn1ooth. Color-Scheele’s 1 Claim. (Cl. 47-61) Green, Plate 860/2 (Wilson). Thorns—-none. Prickles-meme. Hairs-none. Before calyx breaks.—Size—medium small. Form—-— The present invention relates to a new and distinct 15 short pointed; with a conspicuous neck; with variety of rose plant of the hybrid tea class, which was foliaceous appendages on the surface of the bud; originated by me by crossing an unnamed and unpatented with slender foliaceous parts extending beyond the seedling identi?ed in the breeding records of my employer tip of the bud equal to 1A or more of its length. assignee as #40/43, with the variety “Orange Delight,” Color—Scheele’s Green, Plate 860 (Wilson). known in Europe as “Queen Juliana” (Plant Patent No. As calyx breaks.-Color (outside of petal)--Nas 874). turtium Orange, Plate 610/1 (Wilson). The primary objective in making this cross was to pro As ?rst petal opens.—-Size—medium large. Form-— duce a new rose variety, the blooms of which have the short pointed. -

CHROMIX® G and L Admixtures for Color-Conditioned® Concrete
® CHROMIX G and L Admixtures for Color-Conditioned® Concrete n Integral Concrete Color Chart A-350GL n Detroit Riverwalk CHROMIX® G Admixtures for Color-Conditioned® Concrete are free-flowing concentrated pigment granules designed Protect Your Concrete with Scofield Sealers to permanently color concrete and other cementitious Scofield sealers protect the surface, enhance the appearance of colored concrete, and may materials. They can be poured directly into concrete provide protection against contamination and chemical attack by imparting a film-forming layer on the surface of the concrete. Consult a Scofield Concrete Curing & Sealing Guide. mixes, conveyed by gravity feed or pneumatic equipment, or pre-dispensed into pulpable bags. ® Colors shown approximate the color of broom-finished concrete flatwork made with a medium-gray cement and cured with LITHOCHROME Colorwax in the matching color. If Colorwax is not used, the concrete color will not be as vibrant as shown. Concrete should be batched and placed in accordance with Tech-Data Bulletins. CHROMIX G and L Admixtures Concrete color is altered by many factors, including cement and aggregate color, slump, finishing practices, and curing method. Using the contemplated for Color-Conditioned® Concrete materials and construction techniques, representative samples should be cast for approval, especially when exact color matching is important. C-10 Cool Gray C-284 Landmarks Gray C-2436 Flint C-24 Charcoal C-34 Dark Gray C-3650 Navajo Red C-3760 Roman Tile C-3250 Chicory Spice C-3450 Musky