Cenh3 Evolution in Diploids and Polyploids of Three Angiosperm Genera Rick E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
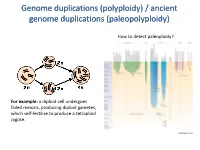
Polyploidy) / Ancient Genome Duplications (Paleopolyploidy
Genome duplications (polyploidy) / ancient genome duplications (paleopolyploidy) How to detect paleoploidy? For example: a diploid cell undergoes failed meiosis, producing diploid gametes, which self-fertilize to produce a tetraploid zygote. Timing of duplication by trees (phylogenetic timing) Phylogenetic timing of duplicates b Paramecium genome duplications Comparison of two scaffolds originating from a common ancestor at the recent WGD Saccharomyces cerevisiae Just before genome duplication Just after genome duplication More time after genome duplication Unaligned view (removing gaps just like in cerev has occurred) Saccharomyces cerevisiae Problem reciprocal gene loss (extreme case); how to solve? Problem reciprocal gene loss (extreme case); how to solve? Just before genome duplication Outgroup! Just after genome duplication Outgroup Just after genome duplication Outgroup More time after genome duplication Outgroup Problem (extreme case); how to solve? Outgroup Outgroup Outgroup Outgroup Outgroup Using other genomes Wong et al. 2002 PNAS Centromeres Vertebrate genome duplication Nature. 2011 Apr 10. [Epub ahead of print] Ancestral polyploidy in seed plants and angiosperms. Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, Depamphilis CW. Flowering plants Flowering MOSS Vertebrates Teleosts S. serevisiae and close relatives Paramecium Reconstructed map of genome duplications allows unprecedented mapping -

Polyploidy and the Evolutionary History of Cotton
POLYPLOIDY AND THE EVOLUTIONARY HISTORY OF COTTON Jonathan F. Wendel1 and Richard C. Cronn2 1Department of Botany, Iowa State University, Ames, Iowa 50011, USA 2Pacific Northwest Research Station, USDA Forest Service, 3200 SW Jefferson Way, Corvallis, Oregon 97331, USA I. Introduction II. Taxonomic, Cytogenetic, and Phylogenetic Framework A. Origin and Diversification of the Gossypieae, the Cotton Tribe B. Emergence and Diversification of the Genus Gossypium C. Chromosomal Evolution and the Origin of the Polyploids D. Phylogenetic Relationships and the Temporal Scale of Divergence III. Speciation Mechanisms A. A Fondness for Trans-oceanic Voyages B. A Propensity for Interspecific Gene Exchange IV. Origin of the Allopolyploids A. Time of Formation B. Parentage of the Allopolyploids V. Polyploid Evolution A. Repeated Cycles of Genome Duplication B. Chromosomal Stabilization C. Increased Recombination in Polyploid Gossypium D. A Diverse Array of Genic and Genomic Interactions E. Differential Evolution of Cohabiting Genomes VI. Ecological Consequences of Polyploidization VII. Polyploidy and Fiber VIII. Concluding Remarks References The cotton genus (Gossypium ) includes approximately 50 species distributed in arid to semi-arid regions of the tropic and subtropics. Included are four species that have independently been domesticated for their fiber, two each in Africa–Asia and the Americas. Gossypium species exhibit extraordinary morphological variation, ranging from herbaceous perennials to small trees with a diverse array of reproductive and vegetative -

A 'David and Goliath'
Current Genomics, 2002, 3, 563-576 563 Nucleolar Dominance: A ‘David and Goliath’ Chromatin Imprinting Process W. Viegas1,*, N. Neves1,2, M. Silva1, A. Caperta1,2, and L. Morais-Cecílio1 1Centro de Botânica Aplicada à Agricultura, Secção de Genética/DBEB, Instituto Superior de Agronomia, 1349-017 Lisboa, 2Departamento de Ciências Biológicas e Naturais, Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-042 Lisboa, Portugal Abstract: Nucleolar dominance is an enigma. The puzzle of differential amphiplasty has remained unresolved since it was first recognised and described in Crepis hybrids by Navashin in 1934. Here we review the body of knowledge that has grown out of the many models that have tried to find the genetic basis for differential rRNA gene expression in hybrids, and present a new interpretation. We propose and discuss a chromatin imprinting model which re-interprets differential amphiplasty in terms of two genomes of differing size occupying a common space within the nucleus, and with heterochromatin as a key player in the scenario. Difference in size between two parental genomes induces an inherited epigenetic mark in the hybrid that allows patterns of chromatin organization to have positional effects on the neighbouring domains. This chromatin imprinting model can be also used to explain complex genomic interactions which transcend nucleolar dominance and which can account for the overall characteristics of hybrids. Gene expression in hybrids, relative to parentage, is seen as being based on the nuclear location of the sequences concerned within their genomic environment, and where the presence of particular repetitive DNA sequences are ‘sensed’, and render silent the adjacent information. -

Gene Loss and Adaptation in Saccharomyces Genomes
Genetics: Published Articles Ahead of Print, published on December 1, 2005 as 10.1534/genetics.105.048900 After the duplication: gene loss and adaptation in Saccharomyces genomes Paul F. Cliften*,1, Robert S. Fulton§, Richard K. Wilson*, §, and Mark Johnston* *Department of Genetics and §Genome Sequencing Center, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO, 63110; 1Current address: Department of Biology, Utah State University, 5305 Old Main Hill, Logan, UT, 84322 Running head: Saccharomyces genomic duplications Key words: genomic duplication, comparative sequence analysis, Saccharomyces phylogeny, yeast Corresponding author: Mark Johnston Department of Genetics Campus Box 8232 Washington University Medical School 4566 Scott Ave. St. Louis, MO 63110 TEL: 314-362-2735 FAX: 314-362-2157 [email protected] ABSTRACT The ancient duplication of the Saccharomyces cerevisiae genome and subsequent massive loss of duplicated genes is apparent when it is compared to the genomes of related species that diverged before the duplication event. To learn more about the evolutionary effects of the duplication event, we compared the S. cerevisiae genome to other Saccharomyces genomes. We demonstrate that the whole genome duplication occurred before S. castellii diverged from S. cerevisiae. In addition to more accurately dating the duplication event, this finding allowed us to study the effects of the duplication on two separate lineages. Analyses of the duplication regions of the genomes indicate that most of the duplicated genes (approximately 85%) were lost before the speciation. Only a small amount of paralogous gene loss (4-6%) occurred after speciation. On the other hand, S. castellii appears to have lost several hundred genes that were not retained as duplicated paralogs. -

Iv Gene Duplication and the Evolution of Silenced
Gene Duplication and the Evolution of Silenced Chromatin in Yeasts by Meleah A. Hickman University Program in Genetics and Genomics Duke University Date:_______________________ Approved: ___________________________ Laura Rusche, Advisor ___________________________ Paul Magwene, Chair ___________________________ Fred Dietrich ___________________________ Joe Heitman ___________________________ Beth Sullivan Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University Program in Genetics and Genomics in the Graduate School of Duke University 2010 i v ABSTRACT Gene Duplication and the Evolution of Silenced Chromatin in Yeasts by Meleah A. Hickman University Program in Genetics and Genomics Duke University Date:_______________________ Approved: ___________________________ Laura Rusche, Advisor ___________________________ Paul Magwene, Chair ___________________________ Fred Dietrich ___________________________ Joe Heitman ___________________________ Beth Sullivan An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University Program in Genetics and Genomics in the Graduate School of Duke University 2010 Copyright by Meleah A. Hickman 2010 Abstract In Saccharomyces cerevisiae, proper maintenance of haploid cell identity requires the SIR complex to mediate the silenced chromatin found at the cryptic mating-type loci, HML and HMR. This complex consists of Sir2, a histone deacetylase, and the histone binding proteins Sir3 and Sir4. Interestingly, both Sir2 and Sir3 have paralogs from a genome duplication that occurred after the divergence of Saccharomyces and Kluyveromyces species, approximately 100 million years ago. The histone deacetylase HST1 is the paralog of SIR2 and works with the promoter-specific SUM1 complex to repress sporulation and alpha-specific genes. ORC1 is the paralog of SIR3 and is an essential subunit of the Origin Recognition Complex and also recruits SIR proteins to the HM loci. -

Whole-Genome Duplications and Paleopolyploidy Whole-Genome Duplications
Whole-genome duplications and paleopolyploidy Whole-genome duplications AUTOPOLYPLOIDY ALLOPOLYPLOIDY Hegarty and HiscockHegarty 2008, 18 Current Biology Examples of allopolyploid speciation Hegarty and Hiscock 2008, Current Biology 18 Whole-genome duplications of different age time neopolyploidy mesopolyploidy paleopolyploidy time Whole-genome duplications in protozoa •Aury et al. (2006) analyzed the unicellular eukaryote Paramecium tetraurelia • most of 40,000 genes arose through at least 3 successive whole-genome duplications (WGDs) • most recent duplication most likely caused an explosion of speciation events that gave rise to the P. aurelia complex (15 sibling species) • some genes have been lost, some retained • many retained (duplicated) genes do not generate functional innovations but are important because of the gene dosage effect Whole-genome duplications in yeast • genome comparison between two yeast species, Saccharomyces cerevisiae (n=16) and Kluyveromyces waltii (n=8) • each region of K. waltii corresponding to two regions of S. cerevisiae •the S. cerevisiae genome underwent a WGD after the two yeast species diverged • in nearly every case (95%), accelerated evolution was confined to only one of the two paralogues (= one of the paralogues retained an ancestral function, the other was free to evolve more rapidly and acquired a derived function) Kellis et al. 2004, Nature 428 Whole-genome duplications in yeast Kellis et al. 2004, Nature 428 a) after divergence from K. waltii, the Saccharomyces lineage underwent a genome duplication event (2 copies of every gene and chromosome) b) duplicated genes were mutated and some lost c) two copies kept for only a small minority of duplicated genes d) the conserved order of duplicated genes (nos. -

Transposable Elements in Polyploid Evolution Final.Pdf
Transposable elements and polyploid evolution in animals Fernando Rodriguez and Irina R. Arkhipova Address Josephine Bay Paul Center for Comparative Molecular Biology and Evolution, Marine Biological Laboratory, Woods Hole, MA 02543, USA Corresponding author: Arkhipova, Irina R. ([email protected]) Polyploidy in animals is much less common than in plants, where it is thought to be pervasive in all higher plant lineages. Recent studies have highlighted the impact of polyploidization and the associated process of diploidy restoration on the evolution and speciation of selected taxonomic groups in the animal kingdom: from vertebrates represented by salmonid fishes and African clawed frogs to invertebrates represented by parasitic root-knot nematodes and bdelloid rotifers. In this review, we focus on the unique and diverse roles that transposable elements may play in these processes, from marking and diversifying subgenome-specific chromosome sets prior to hybridization, to influencing genome restructuring during rediploidization, to affecting subgenome-specific regulatory evolution, and occasionally providing opportunities for domestication and gene amplification to restore and improve functionality. There is still much to be learned from the future comparative genomic studies of chromosome-sized and haplotype-aware assemblies, and from post-genomic studies elucidating genetic and epigenetic regulatory phenomena across short and long evolutionary distances in the metazoan tree of life. Glossary Polyploidy: having more than the usual diploid set of homologous chromosomes Allopolyploidy: a form of polyploidy that results from interspecific hybridization Autopolyploidy: a form of polyploidy that results from chromosome redoubling in the same species Paleopolyploidy: ancient polyploidy Neopolyploidy: recent polyploidy Homeologs: pairs of genes originated by speciation and brought back together by allopolyploidization Ohnologs: same as homeologs, named after S. -

A Global Assembly of Cotton Ests
Downloaded from genome.cshlp.org on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Resource A global assembly of cotton ESTs Joshua A. Udall,1 Jordan M. Swanson,1 Karl Haller,2 Ryan A. Rapp,1 Michael E. Sparks,1 Jamie Hatfield,2 Yeisoo Yu,3 Yingru Wu,4 Caitriona Dowd,4 Aladdin B. Arpat,5 Brad A. Sickler,5 Thea A. Wilkins,5 Jin Ying Guo,6 Xiao Ya Chen,6 Jodi Scheffler,7 Earl Taliercio,7 Ricky Turley,7 Helen McFadden,4 Paxton Payton,8 Natalya Klueva,9 Randell Allen,9 Deshui Zhang,10 Candace Haigler,10 Curtis Wilkerson,11 Jinfeng Suo,12 Stefan R. Schulze,13 Margaret L. Pierce,14 Margaret Essenberg,14 HyeRan Kim,3 Danny J. Llewellyn,4 Elizabeth S. Dennis,4 David Kudrna,3 Rod Wing,3 Andrew H. Paterson,13 Cari Soderlund,2 and Jonathan F. Wendel1,15 1Department of Ecology, Evolution, and Organismal Biology, Iowa State University, Ames, Iowa 50011, USA; 2Arizona Genomics Computational Laboratory, BIO5 Institute, 3Arizona Genomics Institute, Department of Plant Sciences, University of Arizona, Tucson, Arizona 85721, USA; 4CSIRO Plant Industry, Canberra City ACT 2601, Australia; 5Department of Plant Sciences, University of California–Davis, Davis, California 95616, USA; 6Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Shanghai, 200032, China; 7United States Department of Agriculture–Agricultural Research Service, Stoneville, Mississippi 38776, USA; 8United States Department of Agriculture–Agricultural Research Service, Lubbock, Texas 79415, USA; 9Department of Biology, Texas Tech University, -

Review and Advances in Style Curvature for the Malvaceae Cheng-Jiang Ruan*
® International Journal of Plant Developmental Biology ©2010 Global Science Books Review and Advances in Style Curvature for the Malvaceae Cheng-Jiang Ruan* Key Laboratory of Biotechnology & Bio-Resources Utilization, Dalian Nationalities University, Dalian City, Liaoning 116600, China Correspondence : * [email protected] ABSTRACT The flowers of the Malvaceae with varying levels of herkogamy via style curvature have long intrigued evolutionary botanists. This review covers the flower opening process, approach herkogamy, style curvature and character evolution based on molecular phylogenetic trees, adaptive significances of style curvature and the mating system in some portions of the genera in this family. Hermaphroditic flowers of some species have showy petals and pollen and nectar rewards to pollinators. Approach herkogamy, in which stigmas are located on the top of a monadelphous stamen, has evolved as a mechanism to reduce the frequency of intra-floral self-pollination or the interference between male-female organs. Protandrous or monochogamous flowers in the fields open at about 5-7 days and 1-2 days respectively, and pollination is conducted by insects and birds. Interestingly, un-pollinated styles in some species curve when pollination fails. According to our observations and published or internet data, this curvature occurs in 23 species distributed in eight genera of four tribes (Malvavisceae, Ureneae, Hibisceae, Malveae) and appears to have evolved at least eight times. A shift to use style curvature is associated with a shift to annual or perennial herbs, and an unpredictable pollinator environment is likely an important trigger for this evolution. The adaptive significances of style curvature in the Malvaceae include delayed selfing, promotion of outcrossing or reduction in intrafloral male-female interference, sometimes two or three of which simultaneously occur in style curvature of one species (e.g., Kosteletzkya virginica). -

U5b5c4e4 OA.Pdf
Downloaded from genome.cshlp.org on September 9, 2019 - Published by Cold Spring Harbor Laboratory Press Letter Differential lineage-specific amplification of transposable elements is responsible for genome size variation in Gossypium Jennifer S. Hawkins,1 HyeRan Kim,2 John D. Nason,1 Rod A. Wing,2 and Jonathan F. Wendel1,3 1Iowa State University, Department of Ecology, Evolution and Organismal Biology, Ames, Iowa 50011, USA; 2University of Arizona, Department of Plant Sciences, Arizona Genomics Institute, Tucson, Arizona 85721, USA The DNA content of eukaryotic nuclei (C-value) varies ∼200,000-fold, but there is only a ∼20-fold variation in the number of protein-coding genes. Hence, most C-value variation is ascribed to the repetitive fraction, although little is known about the evolutionary dynamics of the specific components that lead to genome size variation. To understand the modes and mechanisms that underlie variation in genome composition, we generated sequence data from whole genome shotgun (WGS) libraries for three representative diploid (n = 13) members of Gossypium that vary in genome size from 880 to 2460 Mb (1C) and from a phylogenetic outgroup, Gossypioides kirkii, with an estimated genome size of 588 Mb. Copy number estimates including all dispersed repetitive sequences indicate that 40%–65% of each genome is composed of transposable elements. Inspection of individual sequence types revealed differential, lineage-specific expansion of various families of transposable elements among the different plant lineages. Copia-like retrotransposable element sequences have differentially accumulated in the Gossypium species with the smallest genome, G. raimondii, while gypsy-like sequences have proliferated in the lineages with larger genomes. -

Comparative Analysis of Gossypium and Vitis Genomes Indicates Genome Duplication Specific to the Gossypium Lineage
Genomics 97 (2011) 313–320 Contents lists available at ScienceDirect Genomics journal homepage: www.elsevier.com/locate/ygeno Comparative analysis of Gossypium and Vitis genomes indicates genome duplication specific to the Gossypium lineage Lifeng Lin a, Haibao Tang a, Rosana O. Compton a, Cornelia Lemke a, Lisa K. Rainville a, Xiyin Wang a,b, Junkang Rong a,1, Mukesh Kumar Rana a,c, Andrew H. Paterson a,⁎ a Plant Genome Mapping Laboratory, University of Georgia, Athens, GA 30605, USA b Center for Genomics and Biocomputing, College of Sciences, Hebei Polytechnic University, Tangshan, Hebei, 063000, China c NRC on DNA Fingerprinting, NBPGR, Pusa Campus, New Delhi, 110012, India article info abstract Article history: Genetic mapping studies have suggested that diploid cotton (Gossypium) might be an ancient polyploid. Received 17 November 2010 However, further evidence is lacking due to the complexity of the genome and the lack of sequence resources. Accepted 15 February 2011 Here, we used the grape (Vitis vinifera) genome as an out-group in two different approaches to further explore Available online 22 February 2011 evidence regarding ancient genome duplication (WGD) event(s) in the diploid Gossypium lineage and its (their) effects: a genome-level alignment analysis and a local-level sequence component analysis. Both Keywords: Gossypium studies suggest that at least one round of genome duplication occurred in the Gossypium lineage. Also, gene Lineage specific genome-wide duplication densities in corresponding regions from Gossypium raimondii, V. vinifera, Arabidopsis thaliana and Carica Vitis vinifera papaya genomes are similar, despite the huge difference in their genome sizes and the different number of Whole genome alignment WGDs each genome has experienced. -

De Novo Genome Sequence Assemblies of Gossypium Raimondii and Gossypium Turneri
GENOME REPORT De Novo Genome Sequence Assemblies of Gossypium raimondii and Gossypium turneri Joshua A. Udall,*,1 Evan Long,† Chris Hanson,† Daojun Yuan,‡ Thiruvarangan Ramaraj,§ Justin L. Conover,‡ Lei Gong,** Mark A. Arick,†† Corrinne E. Grover,‡ Daniel G. Peterson,†† and Jonathan F. Wendel‡ *USDA/Agricultural Research Service, Crop Germplasm Research Unit, College Station, TX 77845, †Plant and Wildlife Science Dept. Brigham Young University, Provo, UT 84042, ‡Ecology, Evolution, and Organismal Biology Dept., Iowa § State University, Ames, IA 50010, School of Computing, DePaul University, Chicago, IL 60604, **Key Laboratory of Molecular Epigenetics of the Ministry of Education, Northeast Normal University, Changchun, China, and ††Institute for Genomics, Biocomputing & Biotechnology, Mississippi State University, Mississippi State, Mississippi 39762 ORCID IDs: 0000-0003-0978-4764 (J.A.U.); 0000-0001-6007-5571 (D.Y.); 0000-0002-7333-1041 (T.R.); 0000-0002-3558-6000 (J.L.C.); 0000-0001-6429-267X (L.G.); 0000-0003-3878-5459 (C.E.G.); 0000-0002-0274-5968 (D.G.P.); 0000-0003-2258-5081 (J.F.W.) ABSTRACT Cotton is an agriculturally important crop. Because of its importance, a genome sequence of a KEYWORDS diploid cotton species (Gossypium raimondii, D-genome) was first assembled using Sanger sequencing Gossypium data in 2012. Improvements to DNA sequencing technology have improved accuracy and correctness of raimondii assembled genome sequences. Here we report a new de novo genome assembly of G. raimondii and its Gossypium close relative G. turneri. The two genomes were assembled to a chromosome level using PacBio long-read turneri technology, HiC, and Bionano optical mapping. This report corrects some minor assembly errors found cotton in the Sanger assembly of G.