Foregut Endoderm Requirement in Neural Patterning 311
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Embryology of Branchial Region
TRANSCRIPTIONS OF NARRATIONS FOR EMBRYOLOGY OF THE BRANCHIAL REGION Branchial Arch Development, slide 2 This is a very familiar picture - a median sagittal section of a four week embryo. I have actually done one thing correctly, I have eliminated the oropharyngeal membrane, which does disappear sometime during the fourth week of development. The cloacal membrane, as you know, doesn't disappear until the seventh week, and therefore it is still intact here, but unlabeled. But, I've labeled a couple of things not mentioned before. First of all, the most cranial part of the foregut, that is, the part that is cranial to the chest region, is called the pharynx. The part of the foregut in the chest region is called the esophagus; you probably knew that. And then, leading to the pharynx from the outside, is an ectodermal inpocketing, which is called the stomodeum. That originally led to the oropharyngeal membrane, but now that the oropharyngeal membrane is ruptured, the stomodeum is a pathway between the amniotic cavity and the lumen of the foregut. The stomodeum is going to become your oral cavity. Branchial Arch Development, slide 3 This is an actual picture of a four-week embryo. It's about 5mm crown-rump length. The stomodeum is labeled - that is the future oral cavity that leads to the pharynx through the ruptured oropharyngeal membrane. And I've also indicated these ridges separated by grooves that lie caudal to the stomodeum and cranial to the heart, which are called branchial arches. Now, if this is a four- week old embryo, clearly these things have developed during the fourth week, and I've never mentioned them before. -

Insect Digestion
INSECT DIGESTION Zoo 514 Dr. Reem Alajmi Insect digestive system The alimentary canal is concerned with digestion and absorption of food stuffs. Different parts of the gut are concerned with different aspects of these functions. In fluid feeding insects digestion begin before the food is ingested through the injection or regurgitation of enzymes on to the food . In general it occurs in the mid gut (most of the enzymes are produced). The enzymes break down the complex substances in the food into simple substances which can be absorbed and assimilated. Enzymes function optimally only within limited range of pH and temperature. Absorption is passive process in some cases , but in others active transport occurs. Absorption of water is important in terrestrial insects , rectum remove water from faeces. • Insects of different groups consume astonishing variety of foods, including watery xylem sap, vertebrate blood, dry wood, bacteria and algae and the internal tissues of other insects. • Types of mouth parts correlates with the diets of insect. Also gut structure and function affected by nutrient composition of the food eaten by insect. • Insects that take solid food typically have a wide, straight, short gut with strong musculature and obvious protection from abrasion (especially in the midgut, which has no cuticular lining). • These features are most obvious in solid-feeders with rapid throughput of food, as in many insects and plant-feeding caterpillars In contrast, insects feeding on blood, sap or nectar usually have long, narrow, convoluted guts to allow maximal contact with the liquid food; here, protection from abrasion is unnecessary. The most obvious gut specialization of liquid-feeders is a mechanism for removing excess water to concentrate nutrient substances prior to digestion, as seen in hemipterans. -
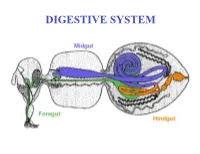
DIGESTIVE SYSTEM Generalized Insect Alimentary Tract the Digestive System Is Just a Tube Within a Surrounding Tube Called the Body
DIGESTIVE SYSTEM Generalized insect alimentary tract The digestive system is just a tube within a surrounding tube called the body. It starts with a mouth and ends with the anus. What goes on in between depends on the insect, its life stage and what it eats. The origin of the digestive tract. At the anterior pole of the embryo an indentation forms that will be the foregut or stomodeum. At the other end a similar thing occurs and the proctodeum or hindgut is formed. Both are lined by cuticle. They both are of ectodermal origin while the midgut is of mesodermal origin and is also called the mesenteron. This different origin of the midgut from the endoderm and not the ecotoderm probably explains why it is not lined with cuticle Anterior midgut invagination. In the bottom photo note the invagination starting forming the ventral furrow lumen (VF) MIDGUT FORMATION IN THE EMBRYO PMG in the above embryo shows the posterior midgut invagination cup where the posterior invagination shown in the drawing on the right will take place. Photo of Drosophila embryo. Hindgut invagination DIGESTIVE SYSTEM The digestive tract not only aids in obtaining, processing and digesting food molecules - It is the largest endocrine tissue in both humans and insects. The digestive system is involved in: 1. Obtaining food 2. Mechanically breaking it down into smaller particles that facilitate digestive enzymes acting on them 3. Enzymatic breakdown of larger food molecules into molecules that can pass through the digestive tract (midgut) and enter the hemolymph 4. Produces molecules or MESSENGERS (eg. -

Embryology and Teratology in the Curricula of Healthcare Courses
ANATOMICAL EDUCATION Eur. J. Anat. 21 (1): 77-91 (2017) Embryology and Teratology in the Curricula of Healthcare Courses Bernard J. Moxham 1, Hana Brichova 2, Elpida Emmanouil-Nikoloussi 3, Andy R.M. Chirculescu 4 1Cardiff School of Biosciences, Cardiff University, Museum Avenue, Cardiff CF10 3AX, Wales, United Kingdom and Department of Anatomy, St. George’s University, St George, Grenada, 2First Faculty of Medicine, Institute of Histology and Embryology, Charles University Prague, Albertov 4, 128 01 Prague 2, Czech Republic and Second Medical Facul- ty, Institute of Histology and Embryology, Charles University Prague, V Úvalu 84, 150 00 Prague 5 , Czech Republic, 3The School of Medicine, European University Cyprus, 6 Diogenous str, 2404 Engomi, P.O.Box 22006, 1516 Nicosia, Cyprus , 4Department of Morphological Sciences, Division of Anatomy, Faculty of Medicine, C. Davila University, Bucharest, Romania SUMMARY Key words: Anatomy – Embryology – Education – Syllabus – Medical – Dental – Healthcare Significant changes are occurring worldwide in courses for healthcare studies, including medicine INTRODUCTION and dentistry. Critical evaluation of the place, tim- ing, and content of components that can be collec- Embryology is a sub-discipline of developmental tively grouped as the anatomical sciences has biology that relates to life before birth. Teratology however yet to be adequately undertaken. Surveys (τέρατος (teratos) meaning ‘monster’ or ‘marvel’) of teaching hours for embryology in US and UK relates to abnormal development and congenital medical courses clearly demonstrate that a dra- abnormalities (i.e. morphofunctional impairments). matic decline in the importance of the subject is in Embryological studies are concerned essentially progress, in terms of both a decrease in the num- with the laws and mechanisms associated with ber of hours allocated within the medical course normal development (ontogenesis) from the stage and in relation to changes in pedagogic methodol- of the ovum until parturition and the end of intra- ogies. -

Facial and Palatal Development
11. FACIAL AND PALATAL DEVELOPMENT Letty Moss-Salentijn DDS, PhD Dr. Edwin S.Robinson Professor of Dentistry (in Anatomy and Cell Biology) E-mail: [email protected] READING ASSIGNMENT: Larsen 3rd edition: p.352; pp.365-371; 398-404. SUMMARY: The external human face develops between the 4th and 6th weeks of embryonic development. Facial swellings arise on the frontonasal process (2 medial nasal and 2 lateral nasal processes) and the first pharyngeal arch (2 mandibular and 2 maxillary processes). By a process of merging and some localized fusion these processes come together to form the continuous surfaces of the external face. The primary palate is formed in this period by fusion/merging of the medial nasal and maxillary processes. Subsequently, between 6th and 12th embryonic/fetal weeks, the secondary palate is formed as the result of fusion between palatal processes growing from the oral surfaces of the maxillary processes. Each merging and fusion site is also the site of a potential facial or palatal cleft. LEARNING OBJECTIVES You should be able to: a. Demonstrate on a frontal image of a human face those parts of the face that are formed by contributions from the frontonasal process and those that are formed by contributions from the first pharyngeal arch. b. Describe the following as to site, composition, time of appearance, and fate: oropharyngeal membrane, oronasal membrane. c. List the derivatives of the four pairs of facial processes and the pair of palatal processes. d. Describe the development of the nose and primary palate. e. Explain the differences between the processes of merging and fusion. -

Pharyngeal Arches. Pharyngeal Pouches
Multimedial Unit of Dept. of Anatomy Jagiellonian University The head and neck regions of a 4-week human embryo somewhat resemble these regions of a fish embryo of a comparable stage of development. This explains the former use of the designation „branchial apparatus” – the adjective „branchial” is derived from the Greek word branchia – the gill. The pharyngeal apparatus consists of: pharyngeal arches pharyngeal pouches pharyngeal grooves pharyngeal membranes The pharyngeal arches begin to develop early in the fourth week as neural crest cells migrate into the future head and neck regions. Drawings illustrating the human pharyngeal apparatus. The first pharyngeal arch (mandibular arch) develops two prominences the maxillary prominence (gives rise to maxilla, zygomatic bone, and squamous part of temporal bone) the mandibular prominence (forms the mandible) Drawings illustrating the human pharyngeal apparatus. Drawings illustrating the human pharyngeal apparatus. Drawings illustrating the human pharyngeal apparatus. Drawing of the head, neck, and thoracic regions of a human embryo (about 28 days), illustrating the pharyngeal apparatus. During the fifth week, the second pharyngeal arch enlarges and overgrows the third and fourth arches, forming an ectodermal depression – the cervical sinus. A - Lateral view of the head, neck, and thoracic regions of an embryo (about 32 days), showing the pharyngeal arches and cervical sinus. B - Diagrammatic section through the embryo at the level shown in A, illustrating growth of the second arch over -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

Structure, Function and Development of the Digestive System in Malacostracan Crustaceans and Adaptation to Different Lifestyles
Cell and Tissue Research (2019) 377:415–443 https://doi.org/10.1007/s00441-019-03056-0 REVIEW Structure, function and development of the digestive system in malacostracan crustaceans and adaptation to different lifestyles Jasna Štrus1 & Nada Žnidaršič1 & Polona Mrak1 & Urban Bogataj1 & Günter Vogt2 Received: 15 January 2019 /Accepted: 9 June 2019 /Published online: 4 July 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The digestive system of the malacostracan crustaceans, namely the decapods, isopods, amphipods and mysids, is among the most complex organ systems of the animal kingdom serving multiple functions such as food processing, absorption and storage of nutrients, synthesis of digestive enzymes and blood proteins, detoxification of xenobiotics and osmoregulation. It is rather well investigated compared to other invertebrates because the Malacostraca include many ecological keystone species and food items for humans. The Decapoda and Peracarida share food processing with chewing and filtering structures of the stomach but differ with respect to morphology and ultrastructure of the digestive glands. In the Peracarida, the digestive glands are composed of few, relatively large lateral caeca, whereas in the Decapoda, hundreds to thousands of blindly ending tubules form a voluminous hepatopancreas. Morphogenesis and onset of functionality of the digestive system strongly depend on the mode of development. The digestive system is early developed in species with feeding planktonic larvae and appears late in species with direct lecithotrophic development. Some structures of the digestive system like the stomach ossicles are rather constant in higher taxa and are of taxonomic value, whereas others like the chewing structures are to some degree adapted to the feeding strategy. -
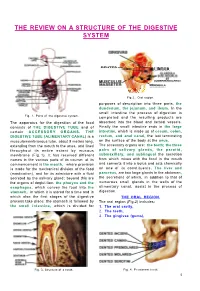
The Review on a Structure of the Digestive System
THE REVIEW ON A STRUCTURE OF THE DIGESTIVE SYSTEM Fig 2.. Oral region. purposes of description into three parts, the duodenum, the jejunum, and ileum. In the small intestine the process of digestion is Fig. 1. Parts of the digestive system. completed and the resulting products are The apparatus for the digestion of the food absorbed into the blood and lacteal vessels. consists of THE DIGESTIVE TUBE and of Finally the small intestine ends in the large certain ACCESSORY ORGANS. THE intestine, which is made up of cecum, colon, DIGESTIVE TUBE (ALIMENTARY CANAL) is a rectum, and anal canal, the last terminating musculomembranous tube, about 9 metres long, on the surface of the body at the anus. extending from the mouth to the anus, and lined The accessory organs are: the teeth; the three throughout its entire extent by mucous pairs of salivary glands, the parotid, membrane (Fig. 1). It has received different submaxillary, and sublingual the secretion names in the various parts of its course: at its from which mixes with the food in the mouth commencement is the mouth, where provision and converts it into a bolus and acts chemically is made for the mechanical division of the food on one of its constituents. The liver and (mastication), and for its admixture with a fluid pancreas, are two large glands in the abdomen, secreted by the salivary gland; beyond this are the secretions of which, in addition to that of the organs of deglutition, the pharynx and the numerous small glands in the walls of the esophagus, which convey the food into the alimentary canal, assist in the process of stomach, in which it is stored for a time and in digestion. -

Respiratory System
Lecture #2 Respiratory system. Development Respiratory system - is a biological system consisting of specific organs and structures used for the process of respiration in an organism Breathing and Respiration BREATHING is the mechanical action of getting air in and out of the lungs. RESPIRATION is the chemical reaction that provides the energy that makes the organism function. It occurs in the cells, more precisely in the mitochondria (the powerplant of the cell). Systema respiratoria Gastrulation – formation of germ layers (4th week) Ectoderm Mesoderm Endoderm Intraembryonic mesoderm plates: • Paraxial (dorsal) mesoderm – axial skeleton (somites) • Intermediate mesoderm – urogenital apparatus • Lateral mesoderm (somatic and splanchnic) – appendicular skeleton and internal organs Coelom Primary gut (foregut) Hollow organs (trachea, bronchi) Intraembryonic cavity Pleural cavity From foregut develop: - Esophagus - Stomach - Duodenum (proximal part) - Liver, pancreas, gall bladder - Respiratory tube Blood supply – truncus coeliacus Sympathetic innervation – n. splanchnicus major Parasympathetic innervation – n.vagus Tubular organ layers development Epithelial lining and glands - Derived from endoderm Lamina propria - Mucosa Muscularis mucosae - Submucosa Derived from visceral mesoderm - Muscularis externa/cartilages - Adventitia/Serosa Development of the upper respiratory system: - nose, nasopharynx, oropharynx Development of the face (from 4th to 8th weeks) 1. Development of the primitive mouth – stomodeum (beginning of the 4th week) 2. Rupture of oropharyngeal membrane (the 24 th day) 3. Development of the nasal cavity (from the end of the 4 th week) 4. Rupture of oronasal membrane (the 6 th week) 5. Development of paranasal air sinuses from diverticuli of nasal walls during late fetal life & after birth Cranial end of the foregut Ratke`s pouch Development of the face (from 4th to 8th weeks) 1. -

Alimentary Canal
Lectures: Po/Monday 9:00 – 10:40 A11 Room 234 Practicals: Út/Tuesday 10:30 – 13:00 Microscopic hall of the Dept. 30 31 32 33 Recommended web-address: http://www.med.muni.cz/histol/vyukac.htm Literature for study: Basic Histology The Developing Human 2008 ISBN: 978-1-4160-3705-7 Before We Are Born, 7th Edition - Essentials of Embryology and Birth Defects With STUDENT CONSULT Online Access By Keith L. Moore, BA, MSc, PhD, FIAC, FRSM and T. V. N. Persaud, MD, PhD, DSc, FRC Path(Lond) 368 pages 1308 ills ($54.95, Softcover) Lecture 1 ESS_3rd semester Outline of development of the digestive system – a revision GENERAL STRUCTURE OF THE ALIMENTARY CANAL (MICROSCOPIC STRUCTURE OF THE ORAL MUCOSA) MICROSCOPIC STRUCTURE OF THE ESOPHAGUS, STOMACH, AND SMALL AND LARGE INTESTINE HISTOPHYSIOLOGY OF THE INTESTINE AND BLOOD CIRCULATION Digestive system consists of the alimentary canal - oral cavity, oropharynx, esophagus, stomach, small and large intestines, rectum and anus associated glands - salivary glands, liver and pancreas function is to obtain from ingested food the metabolites necessary for the growth and energy needs of the body food is digested and transformed into small molecules that can be easily absorbed through the lining of alimentary canal Outline of development of the digestive system Development of the alimentary canal: it constitutes during the 4th week from 3 separate embryonic anlages (organs): the stomodeum (primitive mouth) – develops on the cephalic end of the embryo, is limited by 5 frominences (frontonasal, 2 maxillary, 2 mandibular) -

Embryology20 Dr.Ban
Embryology20 Dr.Ban The midgut Organs in the adult mid gut: Duodenum Jejunum Ileum Cecum Appendix Ascending colon Hepatic flexure of colon Transverse colon (proximal 2/3rd ) The mid gut is the portion of the embryo from which most of the intestine develop. During development, the human mid gut undergoes a rapid phase of growth in which the loop of mid gut ( U shaped loop )herniates outside of the abdominal cavity of the fetus and protrudes into the umbilical cord. This herniation is physiological (occurs normally). • The upper limb of the U is destined to be form the future small intestine • The lower limb forms the ascending and transverse colon. • At the tip of the U, the mid gut is attached to the umbilicus by a thin duct called the vitellointestinal duct which disappears during the later stages of development. • The space between the 2 limbs of the U has the mesentry – a fan shaped structure that holds all the loops of intestine together. 1 Embryology20 Dr.Ban The midgut loops slips back out of the umbilical cord and the physiological hernia ceases to exist. This change coincides with : the termination of the yolk sac and the counter clockwise rotation of the two limbs of the midgut loop around their combined central axis. The U loop undergoes 3 rotations in a step wise manner: First it rotates by 90° in the anticlockwise direction (as seen from the front) along the axis of the superior mesentric artery. At the end of this first rotation the upper limb of the U, or the future ileum comes to lie on the fetus’s right and the lower limb of U or the future colon lies on the left.At the end of 10th week, the midgut retracts back into the abdominal cavity.