Control of Gut Development by Fork Head and Cell Signaling Molecules in Drosophila
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Star Fish Early Development Amphioxus Gastrulation
Faculty of Biological Science and Technology Zoology and Botanical department Practical embryology Amphibian development (4 mm larvae; sagittal sections) By: Shirin Kashfi Ph.D in Animal Development [email protected] tail bud stage At the time of neurulation termination, a part which is known as tail bud develops in the end of embryo body The entire body elongate and shorten in dorso-ventral direction in some extend First muscular responses to external stimuli develop, so this stage refer as muscular response stage too Tail bud is elongated and contains neural tube, notochord and unsegmented mesoderm as well as dorsal and ventral tail fin Five primary brain vesicles are developed (telencephalon, diencephalon, mesencephalon, metencephalon and myelencephalon) Retina (neural retina and retinal pigmented epithelium) and lens placode are formed Auditory vesicle in associated with hind brain and olfactory placode in associated with forebrain (telencephalon) are developed Heart rudiment is developed below to pharynx from mesoderm Foregut, midgut and hindgut are formed. In foregut oral cavity are expanded ventrally and laterally, pharynx and liver diverticulum can be recognizable Pronephric kidney is developed from intermediate mesoderm Somites are formed stage 18, 96 hpf, 4 mm in all sections please consider anterior, posterior, dorsal and ventral directions surface ectoderm yolky endoderm yolky endoderm can be seen in these sections gradually head region is appeared From: embryo/amphibian development/Book - The Frog Its Reproduction -

Embryology of Branchial Region
TRANSCRIPTIONS OF NARRATIONS FOR EMBRYOLOGY OF THE BRANCHIAL REGION Branchial Arch Development, slide 2 This is a very familiar picture - a median sagittal section of a four week embryo. I have actually done one thing correctly, I have eliminated the oropharyngeal membrane, which does disappear sometime during the fourth week of development. The cloacal membrane, as you know, doesn't disappear until the seventh week, and therefore it is still intact here, but unlabeled. But, I've labeled a couple of things not mentioned before. First of all, the most cranial part of the foregut, that is, the part that is cranial to the chest region, is called the pharynx. The part of the foregut in the chest region is called the esophagus; you probably knew that. And then, leading to the pharynx from the outside, is an ectodermal inpocketing, which is called the stomodeum. That originally led to the oropharyngeal membrane, but now that the oropharyngeal membrane is ruptured, the stomodeum is a pathway between the amniotic cavity and the lumen of the foregut. The stomodeum is going to become your oral cavity. Branchial Arch Development, slide 3 This is an actual picture of a four-week embryo. It's about 5mm crown-rump length. The stomodeum is labeled - that is the future oral cavity that leads to the pharynx through the ruptured oropharyngeal membrane. And I've also indicated these ridges separated by grooves that lie caudal to the stomodeum and cranial to the heart, which are called branchial arches. Now, if this is a four- week old embryo, clearly these things have developed during the fourth week, and I've never mentioned them before. -

2/2/2011 1 Development of Development of Endodermal
2/2/2011 ZOO 401- Embryology-Dr. Salah A. Martin DEVELOPMENT OF THE DIGESTIVE SYSTEM ◦ Primitive Gut Tube ◦ Proctodeum and Stomodeum ◦ Stomach Development of Endodermal Organs ◦ Duodenum ◦ Pancreas ◦ Liver and Biliary Apparatus ◦ Spleen ◦ Midgut Wednesday, February 02, 2011 DEVELOPMENT OF THE DIGESTIVE SYSTEM 2 Wednesday, February 02, 2011 Development of Ectodermal Organs 1 ZOO 401- Embryology-Dr. Salah A. Martin ZOO 401- Embryology-Dr. Salah A. Martin Primitive Gut Tube Proctodeum and Stomodeum The primitive gut tube is derived from the dorsal part of the yolk sac , which is incorporated into the body of The proctodeum (anal pit) is the primordial the embryo during folding of the embryo during the fourth week. anus , and the stomodeum is the primordial The primitive gut tube is divided into three sections. mouth . The epithelium of and the parenchyma of In both of these areas ectoderm is in direct glands associated with the digestive tract (e.g., liver and pancreas) are derived from endoderm . contact with endoderm without intervening The muscular walls of the digestive tract (lamina mesoderm, eventually leading to degeneration propria, muscularis mucosae, submucosa, muscularis of both tissue layers. Foregut, Esophagus. externa, adventitia and/or serosa) are derived from splanchnic mesoderm . The tracheoesophageal septum divides the During the solid stage of development the endoderm foregut into the esophagus and of the gut tube proliferates until the gut is a solid tube. trachea. information. A process of recanalization restores the lumen. Wednesday, February 02, 2011 Primitive Gut Tube 3 Wednesday, February 02, 2011 Proctodeum and Stomodeum 4 ZOO 401- Embryology-Dr. Salah A. -

Insect Digestion
INSECT DIGESTION Zoo 514 Dr. Reem Alajmi Insect digestive system The alimentary canal is concerned with digestion and absorption of food stuffs. Different parts of the gut are concerned with different aspects of these functions. In fluid feeding insects digestion begin before the food is ingested through the injection or regurgitation of enzymes on to the food . In general it occurs in the mid gut (most of the enzymes are produced). The enzymes break down the complex substances in the food into simple substances which can be absorbed and assimilated. Enzymes function optimally only within limited range of pH and temperature. Absorption is passive process in some cases , but in others active transport occurs. Absorption of water is important in terrestrial insects , rectum remove water from faeces. • Insects of different groups consume astonishing variety of foods, including watery xylem sap, vertebrate blood, dry wood, bacteria and algae and the internal tissues of other insects. • Types of mouth parts correlates with the diets of insect. Also gut structure and function affected by nutrient composition of the food eaten by insect. • Insects that take solid food typically have a wide, straight, short gut with strong musculature and obvious protection from abrasion (especially in the midgut, which has no cuticular lining). • These features are most obvious in solid-feeders with rapid throughput of food, as in many insects and plant-feeding caterpillars In contrast, insects feeding on blood, sap or nectar usually have long, narrow, convoluted guts to allow maximal contact with the liquid food; here, protection from abrasion is unnecessary. The most obvious gut specialization of liquid-feeders is a mechanism for removing excess water to concentrate nutrient substances prior to digestion, as seen in hemipterans. -

Embryonic Development of the Chicken External Cloaca and Phallus
Scanning Electron Microscopy Volume 1986 Number 2 Article 35 5-23-1986 Embryonic Development of the Chicken External Cloaca and Phallus M. R. Bakst U. S. Department of Agriculture Follow this and additional works at: https://digitalcommons.usu.edu/electron Part of the Biology Commons Recommended Citation Bakst, M. R. (1986) "Embryonic Development of the Chicken External Cloaca and Phallus," Scanning Electron Microscopy: Vol. 1986 : No. 2 , Article 35. Available at: https://digitalcommons.usu.edu/electron/vol1986/iss2/35 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Scanning Electron Microscopy by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. SCANNING ELECTRON MICROSCOPY /1986/11 (Pages 653-659) SEM Inc., AMF O'Hare (Chicago), IL 60666-0507 USA EMBRYONIC DEVELOPMENT OF THE CHICKEN EXTERNAL CLOACA AND PHALLUS M. R. Bakst U.S. Department of Agriculture Agricultural Research Service Avian Physiology Laboratory Beltsville, Maryland 20705 Phone: 301 344 2545 (Received for publication March 22, 1986: revised paper received May 23, 1986) Abstract Introduction The use of the scanning electron microscope In the course of investigating the mechanisms has provided new detailed information about the of erection, ejaculation, and semen dilution in the embryonic development of the chicken external chicken, it was found necessary to determine the cloaca and phallus and has cor:sequently clarified the embryonic origin of the structures in the cloaca origin of the differences between the anatomy of the which form the chicken Phallus nonprotrudens chicken and turkey phallus. -

MINIREVIEW Posterior Gut Development in Drosophila: a Model System for Identifying Genes Controlling Epithelial Morphogen- Esis
Cell Research (1998), 8, 273-284 MINIREVIEW Posterior gut development in Drosophila: a model system for identifying genes controlling epithelial morphogen- esis LENGYEL JUDITH A* , XUE JUN LIU Department of Molecular, Cell and Developmental Biology University of California at Los Angeles, Los Angeles, CA USA, 90095-1606 USA ABSTRACT The posterior gut of the Drosophila embryo, consist- ing of hindgut and Malpighian tubules, provides a simple, well-defined system where it is possible to use a genetic approach to define components essential for epithelial mor- phogenesis. We review here the advantages of Drosophila as a model genetic organism, the morphogenesis of the ep- ithelial structures of the posterior gut, and what is known about the genetic requirements to form these structures. In overview, primordia are patterned by expression of hi- erarchies of transcription factors; this leads to localized expression of cell signaling molecules, and finally, to the least understood step: modulation of cell adhesion and cell shape. We describe approaches to identify additional genes that are required for morphogenesis of these simple epithelia, particularly those that might play a structural role by affecting cell adhesion and cell shape. Key words: Organogenesis, cell rearrangement, con- vergent extension, hindgut, Malpighian tubule. Advantages of Drosophila Work on Drosophila genetics began 90 years ago, when Thomas Hunt Morgan * Corresponding author: [email protected] Drosophila gut epithelial morphogenesis genes (who later received the Nobel Prize for his work) began studying inheritance in the fruit fly. At that time, the advantage of working with this small organism was that it reproduced rapidly in the laboratory, requiring only a simple growth medium, no special attention, and little expense. -
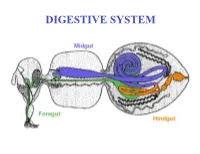
DIGESTIVE SYSTEM Generalized Insect Alimentary Tract the Digestive System Is Just a Tube Within a Surrounding Tube Called the Body
DIGESTIVE SYSTEM Generalized insect alimentary tract The digestive system is just a tube within a surrounding tube called the body. It starts with a mouth and ends with the anus. What goes on in between depends on the insect, its life stage and what it eats. The origin of the digestive tract. At the anterior pole of the embryo an indentation forms that will be the foregut or stomodeum. At the other end a similar thing occurs and the proctodeum or hindgut is formed. Both are lined by cuticle. They both are of ectodermal origin while the midgut is of mesodermal origin and is also called the mesenteron. This different origin of the midgut from the endoderm and not the ecotoderm probably explains why it is not lined with cuticle Anterior midgut invagination. In the bottom photo note the invagination starting forming the ventral furrow lumen (VF) MIDGUT FORMATION IN THE EMBRYO PMG in the above embryo shows the posterior midgut invagination cup where the posterior invagination shown in the drawing on the right will take place. Photo of Drosophila embryo. Hindgut invagination DIGESTIVE SYSTEM The digestive tract not only aids in obtaining, processing and digesting food molecules - It is the largest endocrine tissue in both humans and insects. The digestive system is involved in: 1. Obtaining food 2. Mechanically breaking it down into smaller particles that facilitate digestive enzymes acting on them 3. Enzymatic breakdown of larger food molecules into molecules that can pass through the digestive tract (midgut) and enter the hemolymph 4. Produces molecules or MESSENGERS (eg. -

Evidence for a Thoracic Crop in the Workers of Some Neotropical Pheidole Species (Formicidae: Myrmicinae)
Arthropod Structure & Development 59 (2020) 100977 Contents lists available at ScienceDirect Arthropod Structure & Development journal homepage: www.elsevier.com/locate/asd Evidence for a thoracic crop in the workers of some Neotropical Pheidole species (Formicidae: Myrmicinae) * A. Casadei-Ferreira a, , G. Fischer b, E.P. Economo b a Departamento de Zoologia, Universidade Federal do Parana, Avenida Francisco Heraclito dos Santos, s/n, Centro Politecnico, Curitiba, Mailbox 19020, CEP 81531-980, Brazil b Biodiversity and Biocomplexity Unit, Okinawa Institute of Science and Technology Graduate University, 1919-1 Tancha, Onna, Okinawa, 904-0495, Japan article info abstract Article history: The ability of ant colonies to transport, store, and distribute food resources through trophallaxis is a key Received 28 May 2020 advantage of social life. Nonetheless, how the structure of the digestive system has adapted across the Accepted 21 July 2020 ant phylogeny to facilitate these abilities is still not well understood. The crop and proventriculus, Available online xxx structures in the ant foregut (stomodeum), have received most attention for their roles in trophallaxis. However, potential roles of the esophagus have not been as well studied. Here, we report for the first Keywords: time the presence of an auxiliary thoracic crop in Pheidole aberrans and Pheidole deima using X-ray micro- Ants computed tomography and 3D segmentation. Additionally, we describe morphological modifications Dimorphism Mesosomal crop involving the endo- and exoskeleton that are associated with the presence of the thoracic crop. Our Liquid food results indicate that the presence of a thoracic crop in major workers suggests their potential role as Species group repletes or live food reservoirs, expanding the possibilities of tasks assumed by these individuals in the colony. -

Elixir Journal
45637 Ganesh Elumalai and Jenefa Princess / Elixir Embryology 103 (2017) 45637-45640 Available online at www.elixirpublishers.com (Elixir International Journal) Embryology Elixir Embryology 103 (2017) 45637-45640 “CLOACAL MEMBRANE ANOMALIES” EMBRYOLOGICAL BASIS AND ITS CLINICAL IMPORTANCE Ganesh Elumalai and Jenefa Princess Department of Embryology, College of Medicine, Texila American University, South America. ARTICLE INFO ABSTRACT Article history: Cloacal malformation is a rare but important anomaly. The cloacal anomaly is Received: 1 January 2017; characterised by the persistence of a common channel draining the urinary, genital and Received in revised form: alimentary tracts through a single orifice. It results from abnormal compartmentalization 1 February 2017; of features that are normal in the primitive female embryo. Abnormal embryology and Accepted: 10 February 2017; cloacal anatomy are described in detail. Cloacal abnormalities are usually diagnosed promptly in the neonatal period. Keywords © 2017 Elixir All rights reserved. Cloacal membrane, Uro-rectal septum, Extrophy of the cloaca, Recto-urinary fistulas, Anal agenesis, Rectal atresia. Introduction dilate them to make an anus.. Initial management focuses on Abnormal cloacal development takes place when rectum, anatomic remodelling of the urinary and gastrointestinal vagina and lower urinary tract fuse into a single common system to achieve continence. Improved paediatric channel. Persistent cloaca is a most severe malformation of management strategies have increased the patient survival into cloacal anomalies in girls and is associated with complex adult life. In order to provide appropriate advice, clinicians pelvic malformations. The abnormality of these structures who are undertaking life-long management of adolescent and varies from bladder neck to just beneath the perineal skin. -

A STUDY of ORIGIN, COURSE and VARIATIONS of INFERIOR MESENTERIC ARTERY and ITS BRANCHES Deepa S Ashalatha P R Original Research
Original Research Paper Volume - 7 | Issue - 6 | June - 2017 | ISSN - 2249-555X | IF : 4.894 | IC Value : 79.96 Anatomy A STUDY OF ORIGIN, COURSE AND VARIATIONS OF INFERIOR MESENTERIC ARTERY AND ITS BRANCHES Senior Resident, Department of Anatomy, Government medical college Calicut, Kerala Deepa S 673008 Additional professor, Department of Anatomy, Government medical college, Calicut, Ashalatha P R Kerala 673008 ABSTRACT Knowledge of inferior mesenteric arteries is essential for surgical and radiological procedures. AIM: To study the origin, course, branches and variations of inferior mesenteric arteries. MATERIALS AND METHODS: 50 human cadavers by dissection method. RESULT: Inferior mesenteric artery arose from aorta in all 50 cases. Left colic artery arose from IMA in all 50 cases. Sigmoid arteries arose either directly from IMA or as sigmoid trunk in common with LCA or separately from IMA KEYWORDS : superior mesenteric artery, inferior mesenteric artery, aorta. INTRODUCTION 3. Variations in the branching pattern The mesenteric arterial supply is a combination of rich collateral 4. Presence of any uncommon branches networks and commonly encountered variant anatomy. The effect of normal and variant anatomy has implications on pathology, treatment MATERIALS AND METHODS choices, and planning interventions. A review of anatomic variants The material examined consisted of 50 formalin fixed cadavers will assist in understanding the implications of abnormal anatomy on obtained from the Department of Anatomy, Government Medical treatment for diseases associated with the mesentery. College, Kozhikode. The abdomen was opened by roof top incision. The mesentery of the small intestine in the infracolic compartment was Differences arising during several developmental stages in the exposed by turning the transverse colon and its mesentery upwards. -

Embryology and Teratology in the Curricula of Healthcare Courses
ANATOMICAL EDUCATION Eur. J. Anat. 21 (1): 77-91 (2017) Embryology and Teratology in the Curricula of Healthcare Courses Bernard J. Moxham 1, Hana Brichova 2, Elpida Emmanouil-Nikoloussi 3, Andy R.M. Chirculescu 4 1Cardiff School of Biosciences, Cardiff University, Museum Avenue, Cardiff CF10 3AX, Wales, United Kingdom and Department of Anatomy, St. George’s University, St George, Grenada, 2First Faculty of Medicine, Institute of Histology and Embryology, Charles University Prague, Albertov 4, 128 01 Prague 2, Czech Republic and Second Medical Facul- ty, Institute of Histology and Embryology, Charles University Prague, V Úvalu 84, 150 00 Prague 5 , Czech Republic, 3The School of Medicine, European University Cyprus, 6 Diogenous str, 2404 Engomi, P.O.Box 22006, 1516 Nicosia, Cyprus , 4Department of Morphological Sciences, Division of Anatomy, Faculty of Medicine, C. Davila University, Bucharest, Romania SUMMARY Key words: Anatomy – Embryology – Education – Syllabus – Medical – Dental – Healthcare Significant changes are occurring worldwide in courses for healthcare studies, including medicine INTRODUCTION and dentistry. Critical evaluation of the place, tim- ing, and content of components that can be collec- Embryology is a sub-discipline of developmental tively grouped as the anatomical sciences has biology that relates to life before birth. Teratology however yet to be adequately undertaken. Surveys (τέρατος (teratos) meaning ‘monster’ or ‘marvel’) of teaching hours for embryology in US and UK relates to abnormal development and congenital medical courses clearly demonstrate that a dra- abnormalities (i.e. morphofunctional impairments). matic decline in the importance of the subject is in Embryological studies are concerned essentially progress, in terms of both a decrease in the num- with the laws and mechanisms associated with ber of hours allocated within the medical course normal development (ontogenesis) from the stage and in relation to changes in pedagogic methodol- of the ovum until parturition and the end of intra- ogies. -

Endoderm and Extraembryonic Structures
The Endoderm and Extraembryonic Structures Endoderm: Linings of a Tube • Divides into foregut, midgut, and hindgut • Openings to yolk sac are intestinal portals that close to middle to form yolk stalk Gut Regions How do the Ends Form? • Endodermal openings are stomodeum and proctodeum • Endoderm meets invagination of ectoderm What Comes from Foregut? • Foregut forms pharyngeal pouches, body tongue, thyroid, trachea, lung The Lungs • Lung develops by endothelial branching also typical of many glands • Depends on mesenchyme No mesenchyme--Mesenchyme What Comes from Foregut? • Pharyngeal region forms gills, eardrums, parathyroid, thymus • Breaks through to form gill slits with ectoderm • Connective tissue (cartilage) from neural crest The Pharynx Pouches and arches Further Down Liver and Pancreas • Linings from endoderm • Connective tissue from splanchnic mesoderm Amniotes Have Four Extra- embryonic “Membranes” • Amnion - maintains aqueous environment – amniote vertebrates • Chorion - gas exchange – in mammals --> placenta – also provides nutrition, hormones, immunity • Allantoic membrane - waste disposal/respiration – not necessary in humans because of placenta • Yolk Sac - nutrition – no yolk in humans (yolk sac holds primordial germ cells) Four “Membranes” Where do Membranes Originate? • Chorion and amnion from ectoderm and somatic mesoderm – = body wall or somatopleure • Allantois and yolk sac from endoderm and splanchnic mesoderm – = gut wall or splanchnopleure Extraembryonic Membranes • Membranous folds gradually separate embryo from the extraembryonic regions • Ectoderm + Mesoderm: – Amnion Somatopleure – Chorion (body wall) • Endoderm + Mesoderm: – Yolk sac Splanchnopleure – Allantois (gut wall) And More Folding The Caudal Region .