MINIREVIEW Posterior Gut Development in Drosophila: a Model System for Identifying Genes Controlling Epithelial Morphogen- Esis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elixir Journal
45637 Ganesh Elumalai and Jenefa Princess / Elixir Embryology 103 (2017) 45637-45640 Available online at www.elixirpublishers.com (Elixir International Journal) Embryology Elixir Embryology 103 (2017) 45637-45640 “CLOACAL MEMBRANE ANOMALIES” EMBRYOLOGICAL BASIS AND ITS CLINICAL IMPORTANCE Ganesh Elumalai and Jenefa Princess Department of Embryology, College of Medicine, Texila American University, South America. ARTICLE INFO ABSTRACT Article history: Cloacal malformation is a rare but important anomaly. The cloacal anomaly is Received: 1 January 2017; characterised by the persistence of a common channel draining the urinary, genital and Received in revised form: alimentary tracts through a single orifice. It results from abnormal compartmentalization 1 February 2017; of features that are normal in the primitive female embryo. Abnormal embryology and Accepted: 10 February 2017; cloacal anatomy are described in detail. Cloacal abnormalities are usually diagnosed promptly in the neonatal period. Keywords © 2017 Elixir All rights reserved. Cloacal membrane, Uro-rectal septum, Extrophy of the cloaca, Recto-urinary fistulas, Anal agenesis, Rectal atresia. Introduction dilate them to make an anus.. Initial management focuses on Abnormal cloacal development takes place when rectum, anatomic remodelling of the urinary and gastrointestinal vagina and lower urinary tract fuse into a single common system to achieve continence. Improved paediatric channel. Persistent cloaca is a most severe malformation of management strategies have increased the patient survival into cloacal anomalies in girls and is associated with complex adult life. In order to provide appropriate advice, clinicians pelvic malformations. The abnormality of these structures who are undertaking life-long management of adolescent and varies from bladder neck to just beneath the perineal skin. -

A STUDY of ORIGIN, COURSE and VARIATIONS of INFERIOR MESENTERIC ARTERY and ITS BRANCHES Deepa S Ashalatha P R Original Research
Original Research Paper Volume - 7 | Issue - 6 | June - 2017 | ISSN - 2249-555X | IF : 4.894 | IC Value : 79.96 Anatomy A STUDY OF ORIGIN, COURSE AND VARIATIONS OF INFERIOR MESENTERIC ARTERY AND ITS BRANCHES Senior Resident, Department of Anatomy, Government medical college Calicut, Kerala Deepa S 673008 Additional professor, Department of Anatomy, Government medical college, Calicut, Ashalatha P R Kerala 673008 ABSTRACT Knowledge of inferior mesenteric arteries is essential for surgical and radiological procedures. AIM: To study the origin, course, branches and variations of inferior mesenteric arteries. MATERIALS AND METHODS: 50 human cadavers by dissection method. RESULT: Inferior mesenteric artery arose from aorta in all 50 cases. Left colic artery arose from IMA in all 50 cases. Sigmoid arteries arose either directly from IMA or as sigmoid trunk in common with LCA or separately from IMA KEYWORDS : superior mesenteric artery, inferior mesenteric artery, aorta. INTRODUCTION 3. Variations in the branching pattern The mesenteric arterial supply is a combination of rich collateral 4. Presence of any uncommon branches networks and commonly encountered variant anatomy. The effect of normal and variant anatomy has implications on pathology, treatment MATERIALS AND METHODS choices, and planning interventions. A review of anatomic variants The material examined consisted of 50 formalin fixed cadavers will assist in understanding the implications of abnormal anatomy on obtained from the Department of Anatomy, Government Medical treatment for diseases associated with the mesentery. College, Kozhikode. The abdomen was opened by roof top incision. The mesentery of the small intestine in the infracolic compartment was Differences arising during several developmental stages in the exposed by turning the transverse colon and its mesentery upwards. -

Pattern of Autonomic Innervation
Pattern of Innervation of the Viscera The nerves supplying the viscera are derived from the autonomic nervous system – sympathetic and parasympathetic. The viscera of the thorax, abdomen and pelvis are supplied segmentally by branches of the sympathetic chain, and branches of the vagus nerve, as discussed below. The autonomic supply of structures in the head and neck follows a specific pattern that will be dealt with separately. The sympathetic chain: Communicates with the spinal nerves via white and grey rami communicantes The white rami communicantes: are present only at the levels of T1 to L2 spinal nerves convey all the sympathetic efferents from the spinal cord to the sympathetic chain consist of myelinated pre-ganglionic axons have their cell bodies in the lateral grey column of the spinal cord synapse in the sympathetic ganglia with postganglionic neurons NOTE: The cervical, lower lumbar and sacral ganglia have no white rami, and receive their sympathetic inflow from the thoracic and lumbar segments via ascending or descending axons in the sympathetic chain The grey rami communicantes consist of unmyelinated postganglionic neurons are the axons of postganglionic neurons distribute somatic branches via the cutaneous nerves to the arteries, sweat glands and arrectores pilorum in the skin, (where they are vasoconstrictor, secretomotor, and pilomotor respectively. are present in all spinal ganglia Visceral sympathetic branches are distributed to the viscera via autonomic nerve plexuses (listed in the table below) arise as -

Hadeel Abdullah Nadeen AL-Falooji Dena Kofahi Mohammad Hesham
Hadeel Abdullah Nadeen AL-Falooji Dena Kofahi Mohammad Hesham 0 | P a g e Nerves ON THE POSTERIOR ABDOMINAL WALL The Lumbar Plexus The lumbar plexus, which is one of the main nervous pathways supplying the lower limb, is formed in the psoas major muscle -in the abdomen- from the anterior rami of the upper four lumbar spinal nerves (L1-L4). Its branches emerge from the lateral₁ and medial₂ borders of the muscle and from its anterior₃ surface. Branches of the Lumbar Plexus - The iLiohypogastric nerve₁, iLioinguinal nerve₂, Lateral cutaneous nerve of the thigh₃, and femoraL nerve₄ emerge from the Lateral border of the psoas, in that order from above downward. - The obturator nerve₁ and lumbosacral trunk₂ emerge from the medial border of the psoas major. - The genitofemoral₁ nerve emerges from the anterior surface of the psoas major. The lumbosacral trunk: It is formed from the L4 (from the lumbar plexus) and L5 (from the sacral plexus) nerve roots (i.e. the fourth lumbar nerve gives off branches to the sacral plexus forming the lumbosacral trunk). - The ventral (anterior) rami of L1 form the iliohypogastric₁ and ilioinguinal₂ nerves which run between the transversus abdominis muscle and abdominal internal oblique muscle. Then: 1. The iliohypogastric nerve (L1) gives off several motor branches to abdominal muscles and a sensory branch to the skin of the lower part of the anterior abdominal wall above the pubic symphysis. 2. The ilioinguinal nerve (L1) pierces the posterior wall of the inguinal canal and runs along with the spermatic cord (through the canal) to supply the skin of the groin and the scrotum or labium majus. -

Control of Gut Development by Fork Head and Cell Signaling Molecules in Drosophila
ELSEVIER Mechanisms of Development 58 (1996) 3-14 Control of gut development by fork head and cell signaling molecules in Drosophila Michael Hoch*, Michael J. Pankratz Mar-Plunck-lnstitut,~r Biophysikalische Chemie, Abteilung Molekulare Entwicklungsbiologie, Am Fassberg. 37077 Gdttingen, Germany Received 14 March 1996; accepted 9 April 1996 Abstract The alimentary canal of most animals can be subdivided into a fore-, mid- and hindgut portion, each gut part possessing distinct physiological functions. The genetic basis underlying the formation of the different gut parts is poorly understood. Here we show that the Drosophila genes hedgehog, wingless and decapentuplegic, which encode cell signaling molecules, are required for the establish- ment of signaling centers that coordinate morphogenesis in the hindgut epithelium. The activation of these genes in the developing as well as in the foregut requires fork head, which encodes a transcription factor. Furthermore, we demonstrate that hedgehog and win- gless activities in the gut epithelial cells are required for the expression of the homeobox gene bagpipe in the ensheathing visceral mesoderm. These results provide strong evidence that similar principles underlie Drosophila fore- and hindgut development, and that the genetic hierarchy of gut development might be conserved between Drosophila and vertebrates. Keywords: Cell signaling in the gut; hedgehog; wingless; decapentaplegic; fork head; bagpipe; Gut; Morphogenesis 1. Introduction a continuous gut tube is generated. Regional specification then occurs and several gut-associated organs are formed Nutrition and hydration are basic needs of all organ- at the junction between the different gut parts. isms. The organ which is required to fulfill these needs in The genetic basis underlying gut development is only animals is the gut, which most likely belongs to the most poorly understood. -
Development of the Gastrointestinal Tract and the Urogenital System and Malformations
Development of the gastrointestinal tract and the urogenital system and malformations for pharmacists Semmelweis University, Department of Anatomy, Histology and Embryology 2017. 04.03. by Krisztina H.-Minkó Folding of the Embryo (cross section) https://www.youtube.com/watch?v=qMnpxP6EeIY 4th week Folding of the Embryo (Longitudinal section) Hindgut Foregut Posterior opening of Anterior opening Caudal gut gut Notochord of gut Cranial gut Body stalk Midgut Vitelline duct Allantois Stomodeum + Cloacal Heart anlage membrane buccopharyngeal membrane Folding of the Embryo (Intraembryonic Mesoderm) Dorsal aorta Mesonephros Dorsal mesogastrium Intraembryonal coelom Somites Gut tube Neural tube Visceral mesoderm Notochord Ventral Dorsal aorta mesogastrium Mesonephros Genital crest Gut tube Coelom Intraembryonal coelom Ventral mesentery Dorsal mesentery The endodermal gut tube created by body folding during the 4th week consists of a blindended cranial foregut, a Primitive Gut Tube blind-ended caudal hindgut, and a midgut open to the yolk sac through the vitelline duct. Pharyngeal gut Buccopharyngeal Thyreoglossal membrane duct Yolk sac Esophagus Foregut Heart Lung Vitelline duct Stomach Duodenojejunal Body stalk flexure Midgut Caudal gut Cecum Left colic Cloacal membrane flexure Allantois Cloaca Hindgut Development of GI tract https://www.youtube.com/watch?v=tx3Cn8g-_e0 Vascularisation of the primitive gut tube The arterial supply to the gut develops through consolidation and reduction of the ventral branches of the dorsal aortae that anastomose with the vessel plexuses originally supplying blood to the yolk sac. About five of these vitelline artery derivatives vascularize the thoracic foregut, and three—the celiac, superior mesenteric, and inferior mesenteric arteries—vascularize the abdominal gut. By convention, the boundaries of the foregut, midgut, and hindgut portions of the abdominal gut tube are determined by the respective territories of these three arteries. -

The Parasympathetic System
DR MOUIN ABBOUD PR OF ANATOMY In faculity of medecin ( Damascus and Sham uneversities ) Specialist in respiratory diseases الدكتور معين عبود استاذ التشريح في كلية الطب البشري في جامعة دمشق وجامعة الشام الخاصة اختصاصي في أمراض جهاز التنفس DR MOUIN ABBOUD Abdominal viscera Innervation The Innervation Abdominal viscera are innervated by both : extrinsic ) visceral innervation ( involves : . receiving motor impulses from the central nervous system . and sending sensory information to, the central nervous system; and intrinsic components of the nervous system: involves the regulation of digestive tract activities by a generally self-sufficient network of sensory and motor neurons (the enteric nervous system). Visceral innervation The visceral innervation is transmited by Autonomic Plexuses )prevertebral plexus ). By which : these viscera send sensory information back to the central nervous system through visceral afferent fibers and receive motor impulses from the central nervous system through visceral efferent fibers. prevertebral plexus The abdominal prevertebral plexus receives: preganglionic parasympathetic and visceral afferent fibers from the vagus nerves [X]; preganglionic sympathetic and visceral afferent fibers from the thoracic and lumbar splanchnic nerves; preganglionic parasympathetic fibers from the pelvic splanchnic nerves. The Sympathetic Division The sympathetic division consists of the following: Preganglionic fibers in the lateral grey column of the thoracic and upper two lumbar segments of the spinal cord. Ganglionic neurons in : . Sympathetic chain ganglia, also called paravertebral, or lateral ganglia . Collateral ganglia, also known as prevertebral ganglia . Specialized neurons in the interior of the suprarenal gland Postganglionic fibers : to target organs Sectional Organization of the Spinal Cord The parasympathetic system The parasympathetic system is less neatly defined Preganglionic fibers . -

ULTRA MORPHOLOGY of the DIGESTIVE SYSTEM of Anastrepha Fraterculus and Ceratitis Capitata (DIPTERA TEPHRITIDAE)
REGULAR PAPER ULTRA MORPHOLOGY OF THE DIGESTIVE SYSTEM OF Anastrepha fraterculus AND Ceratitis capitata (DIPTERA TEPHRITIDAE) Flávio Henrique Caetano1, Vera Nisaka Solferini2, Fabio Barros de Britto1, Daniela Silva Lins2, Tamara Aluani2, Vinicius Garcia de Brito2 and Fernando José Zara3 1Department of Biology, Institute of Biosciences, Paulista State University (UNESP), Rio Claro, SP, 2Department of Genetics and Evolution, Institute of Biology, State University of Campinas (UNICAMP), Campinas, SP, 3Paulista State University (UNESP), Coastal Campus, São Vicente, SP, Brazil. ABSTRACT Anastrepha fraterculus and Ceratitis capitata are widely distributed fruit flies that cause significant damage to fruit crops in tropical and temperate regions. The economic importance of these flies has resulted in numerous studies of their biology, with particular emphasis on their control and management. However, various aspects of the biology of these species are still poorly understood. In this work, we used scanning electron microscopy (SEM) to examine the external anatomy and organization of the digestive system in these two species. Adult males and females of A. fraterculus and females of C. capitata were dissected in physiological saline solution, and the digestive tracts were removed and prepared for microscopy. SEM showed that the crop was covered by a strong muscular layer that consisted of circular fibers connected by longitudinal fibers; this arrangement was probably related to the post-feeding behavior of these flies in which the crop contents are regurgitated and reingested. The size of the rectum varied and was probably related to the different body sizes of the two species. Key words: Gut anatomy, scanning electron microscopy, Tephritidae INTRODUCTION that would otherwise be discharged along with the The digestive tract of insects is a continuous feces [4,6,9-11,22]. -

Insect Morphology - Digestive System 1
INSECT MORPHOLOGY - DIGESTIVE SYSTEM 1 * The digestive system is also commonly referred to as the alimentary canal. The alimentary canal is a tube, usually coiled, which extends from the mouth to the anus, and consists of three regions: the foregut, midgut, and hindgut. The foregut is also known as the stomodaeum; the midgut is also known as the mesenteron; and the hindgut is also known as the proctodaeum. Each of these regions may be divided into two or more subregions; and there are usually valves and sphincters between these regions which regulate the passage of food from one region to another. As we mentioned in our last lecture the stomodaeum and the proctodaeum are ectodermal in origin and the mesenteron is endodermal in origin. PREORAL CAVITY * The mouthparts lie close together and form a small cavity often called the mouth cavity. Since it lies anterior to the true opening to the stomodaeum it is more properly known as the preoral cavity [Snodgrass]. The true mouth is the opening of the stomodaeum. This preoral food chamber is also sometimes called the cibarium. There are usually muscles attached to the cibarium that have their other origins inserted on the clypeus (they are located anterior or ventral to the frontal ganglion) - these are called the dilators of the cibarium. FOREGUT * Since the foregut is ectodermal in origin it is lined with a layer of cuticle, known as the intima, which is shed at each molt in the same way as the rest of the cuticle. The foregut epithelium consists of flattened cells with indistinct boundaries. -
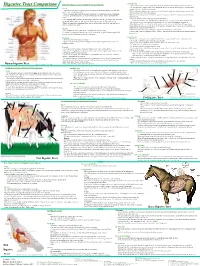
Digestive Tract Comparison • the Small Intestine Is a Tube Roughly Twenty Feet Long Deided Into the Duodenum, Jejunum and Ileum
• Small Intestine Human/Dog Digestive system or Simple Monogastric Digestion Digestive Tract Comparison • The small intestine is a tube roughly twenty feet long deided into the duodenum, jejunum and ileum. • The first part of the small intestine is the duodenum, the site of most chemical digestive reactions and is Mouth smoother than the rest of the small intestine • A specialized region of the digestive tract designed to break up large particles of food into • Bile, bicarbonate and pancreatic enzymes are secreted into the duodenum to breakdown nutrients in the smaller, more manageable particles chyme so that they can be readily absorbed. • Saliva is added to moisten food and begin carbohydrate breakdown by amylase in humans. •Bicarbonate from the pancreas neutralizes corrosive stomach acid from 3.5 in the stomach to 8.5 in the • There are four main types of teeth in the human or dog: incisors, canines, premolars and small intestine. molars. •Pancreatic enzymes include lipases, peptidases and amylases. •One reason dog and cat canines are much larger than ours is that they need to be able to rip and •Lipases break down fats. Peptidases break down proteins. Amylases break down carbohydrates. tear through tough raw meat. Humans have evolved to eat easier to chew, cooked meat. • Bile from the liver is stored in the gall bladder and secreted into the duodenum to emulsify fat. • While chewing, food is transformed into what is called a bolus, a food ball, and then forced •The jejunum and ileum are next in the small intestine and are covered in villi, finger-like projections. -

STRUCTURE and FUNCTIONS of DIGESTIVE SYSTEM The
STRUCTURE AND FUNCTIONS OF DIGESTIVE SYSTEM The alimentary canal of insects is a long, muscular, and tubular structure extending from mouth to anus. It is differentiated into three regions viz., Foregut, Midgut and Hindgut. I. FOREGUT Foregut is ectodermal in origin. Anterior invagination of ectoderm forms foregut (Stomodeum). Internal cuticular lining is present. Terminal mouth parts leads into a preoralcavity. Preoralcavity between epipharynx and hypopharynx is called as Cibarium. Preoralcavity between hypopharynx and salivary duct is Salivarium. Behind the mouth a well musculated organ called Pharynx is present which pushes the food into oesophagous. Pharynx acts as a sucking pump in sap feeders. Oesophagous is a narrow tube which conducts food into crop. Crop is the dilated distal part of oesophagus acting as food reservoir. In bees crop is called as honey stomach where nectar conversion occurs. Proventriculus or Gizzard is the posterior part of foregut and is musculated. It is found in solid feeders and absent in fluid feeders or sap feeders. The internal cuticle of gizzard is variously modified as follows. i. Teeth like in cockroach to grind and strain food. ii. Plate like in honey bee to separate pollen grains from nectar iii. Spine like in flea to break the blood corpuscles Food flow from foregut to midgut is regulated through Cardiac valve or Oesophageal valve. II. MIDGUT Midgut is endodermal in origin and also called as mesentron. This part contains no cuticular lining. Midgut is made up of three types of epithelial cells. (i) Secretory cells (Columnar cells) (ii) Goblet cells (aged secretory cells), (iii) Regenerative cells which replaces secretory cells. -

Endodermal Derivatives, Formation of the Gut and Its Subsequent Rotation
18. ENDODERMAL DERIVATIVES, FORMATION OF THE GUT AND ITS SUBSEQUENT ROTATION Dr. Mike Gershon Department of Anatomy & Cell Biology Telephone: 305-3447 E-mail: [email protected] READING ASSIGNMENT: Larsen 3rd edition: Review Chapter 6: pp. 134—149; Chapter 9, pp. 235-259. SUMMARY:The gut is formed as a critical byproduct of the folding of the germ disc. The primitive bowel extends from the buccopharyngeal membrane to the cloacal membrane. It is portioned into a foregut, a midgut, and a hindgut. The foregut, which gives rise to the largest number of structures forms the pharynx and its derivatives, the lower respiratory tract, the esophagus, the stomach, the duodenum, proximal to the ampulla of Vater, the liver, the pancreas, and the biliary apparatus. From the stomach on, these structures are supplied by the celiac artery. The midgut is supplied by the superior mesenteric artery, and the hindgut is supplied by the inferior mesenteric artery. These vessels define the segmentation of the bowel. The stomach rotates 90° clockwise as it grows, which has major consequences for the positioning of the mesenteries, the duodenum, bile duct, and pancreas. The liver and biliary system arise from the hepatic diverticulum, which grows into the ventral mesentery and septum transversum. The hepatic diverticulum divides into a cranial and a caudal division: the cranial forms the liver and the smaller, caudal forms the extrahepatic biliary system. The pancreas arises from dorsal and ventral pancreatic buds. The rotation of the stomach brings the buds together so that they can fuse into a definitive pancreas. The midgut grows rapidly and herniates into the umbilical cord, where it rotates 90° counterclockwise.