PHARYNGEAL ARCHES & POUCHES Objectives by the End of This Lesson You Should Be Able To: 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Induction and Specification of Cranial Placodes ⁎ Gerhard Schlosser
Developmental Biology 294 (2006) 303–351 www.elsevier.com/locate/ydbio Review Induction and specification of cranial placodes ⁎ Gerhard Schlosser Brain Research Institute, AG Roth, University of Bremen, FB2, PO Box 330440, 28334 Bremen, Germany Received for publication 6 October 2005; revised 22 December 2005; accepted 23 December 2005 Available online 3 May 2006 Abstract Cranial placodes are specialized regions of the ectoderm, which give rise to various sensory ganglia and contribute to the pituitary gland and sensory organs of the vertebrate head. They include the adenohypophyseal, olfactory, lens, trigeminal, and profundal placodes, a series of epibranchial placodes, an otic placode, and a series of lateral line placodes. After a long period of neglect, recent years have seen a resurgence of interest in placode induction and specification. There is increasing evidence that all placodes despite their different developmental fates originate from a common panplacodal primordium around the neural plate. This common primordium is defined by the expression of transcription factors of the Six1/2, Six4/5, and Eya families, which later continue to be expressed in all placodes and appear to promote generic placodal properties such as proliferation, the capacity for morphogenetic movements, and neuronal differentiation. A large number of other transcription factors are expressed in subdomains of the panplacodal primordium and appear to contribute to the specification of particular subsets of placodes. This review first provides a brief overview of different cranial placodes and then synthesizes evidence for the common origin of all placodes from a panplacodal primordium. The role of various transcription factors for the development of the different placodes is addressed next, and it is discussed how individual placodes may be specified and compartmentalized within the panplacodal primordium. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Embryology of Branchial Region
TRANSCRIPTIONS OF NARRATIONS FOR EMBRYOLOGY OF THE BRANCHIAL REGION Branchial Arch Development, slide 2 This is a very familiar picture - a median sagittal section of a four week embryo. I have actually done one thing correctly, I have eliminated the oropharyngeal membrane, which does disappear sometime during the fourth week of development. The cloacal membrane, as you know, doesn't disappear until the seventh week, and therefore it is still intact here, but unlabeled. But, I've labeled a couple of things not mentioned before. First of all, the most cranial part of the foregut, that is, the part that is cranial to the chest region, is called the pharynx. The part of the foregut in the chest region is called the esophagus; you probably knew that. And then, leading to the pharynx from the outside, is an ectodermal inpocketing, which is called the stomodeum. That originally led to the oropharyngeal membrane, but now that the oropharyngeal membrane is ruptured, the stomodeum is a pathway between the amniotic cavity and the lumen of the foregut. The stomodeum is going to become your oral cavity. Branchial Arch Development, slide 3 This is an actual picture of a four-week embryo. It's about 5mm crown-rump length. The stomodeum is labeled - that is the future oral cavity that leads to the pharynx through the ruptured oropharyngeal membrane. And I've also indicated these ridges separated by grooves that lie caudal to the stomodeum and cranial to the heart, which are called branchial arches. Now, if this is a four- week old embryo, clearly these things have developed during the fourth week, and I've never mentioned them before. -

Loss-Of-Function Mutation in the Prokineticin 2 Gene Causes
Loss-of-function mutation in the prokineticin 2 gene SEE COMMENTARY causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism Nelly Pitteloud*†, Chengkang Zhang‡, Duarte Pignatelli§, Jia-Da Li‡, Taneli Raivio*, Lindsay W. Cole*, Lacey Plummer*, Elka E. Jacobson-Dickman*, Pamela L. Mellon¶, Qun-Yong Zhou‡, and William F. Crowley, Jr.* *Reproductive Endocrine Unit, Department of Medicine and Harvard Reproductive Endocrine Science Centers, Massachusetts General Hospital, Boston, MA 02114; ‡Department of Pharmacology, University of California, Irvine, CA 92697; §Department of Endocrinology, Laboratory of Cellular and Molecular Biology, Institute of Molecular Pathology and Immunology, University of Porto, San Joa˜o Hospital, 4200-465 Porto, Portugal; and ¶Departments of Reproductive Medicine and Neurosciences, University of California at San Diego, La Jolla, CA 92093 Communicated by Patricia K. Donahoe, Massachusetts General Hospital, Boston, MA, August 14, 2007 (received for review May 8, 2007) Gonadotropin-releasing hormone (GnRH) deficiency in the human associated with KS, although no functional data on the mutant presents either as normosmic idiopathic hypogonadotropic hypo- proteins were provided (17). Herein, we demonstrate that homozy- gonadism (nIHH) or with anosmia [Kallmann syndrome (KS)]. To gous loss-of-function mutations in the PROK2 gene cause IHH in date, several loci have been identified to cause these disorders, but mice and humans. only 30% of cases exhibit mutations in known genes. Recently, murine studies have demonstrated a critical role of the prokineticin Results pathway in olfactory bulb morphogenesis and GnRH secretion. Molecular Analysis of PROK2 Gene. A homozygous single base pair Therefore, we hypothesize that mutations in prokineticin 2 deletion in exon 2 of the PROK2 gene (c.[163delA]ϩ [163delA]) (PROK2) underlie some cases of KS in humans and that animals was identified in the proband, in his brother with KS, and in his deficient in Prok2 would be hypogonadotropic. -

Development and Malformations of Face and Palate
Development and malformations of face and palate Compiled by András Csillag and Andrea D. Székely GERMINAL LAYER DERIVATIVES ECTODERM contributing to the formation of the face appears by the 4th week. The oropharyngeal membrane (interface between ECTODERM and ENDODERM) is located in front of the later palatine tonsils. Ectodermal structures limiting the stomodeum participate in the formation of the face, as well as of the nasal and oral cavities. MESENCHYME that fills the pharyngeal arches derives from the neural crest ECTOMESENCHYME DEVELOPMENT OF THE FACE WEEK 6 The face is formed by 5 processes approximately 24 days Frontonasal prominence (1) Maxillary prominence (2) 1st arch Mandibular prominence (2) nasal (olfactory) pits form surrounded by the medial and lateral nasal processes nasolacrimal groove separates the lateral nasal process from the maxillary process maxillary processes fuse with the medial nasal processes lateral nasal processes fuse with the maxillary processes, thus obliterating the nasolacrimal groove. DEVELOPMENT OF THE FACE 5-week embryo 6-week embryo 7-week embryo. 10-week embryo The nasal prominences are gradually Maxillary prominences have fused separated from the maxillary with the medial nasal prominences. prominence by deep furrows. CEPHALIC PRIMORDIA - PLACODES Olfactory placode Pharyngeal Stomodeum” arches Nasal placodes - thickenings of the surface ectoderm (later differentiate into the olfactory epithelium) DEVELOPMENT OF THE NASAL CAVITIES AND THE HARD PALATE By week 5 the placodes form the nasal pits. They further invaginate and the pits approach the primitive oral cavity. A thin oronasal membrane separates the two cavities. By its rupture the primitive choanae will form DEVELOPMENT OF THE NASAL CAVITIES AND THE HARD PALATE By week 8, a partition forms between the primitive nasal chambers and the oral cavity The primary palate - the anterior aspect derives from the intermaxillary segment (or median palatine process, formed by the medial nasal processes). -

Insect Digestion
INSECT DIGESTION Zoo 514 Dr. Reem Alajmi Insect digestive system The alimentary canal is concerned with digestion and absorption of food stuffs. Different parts of the gut are concerned with different aspects of these functions. In fluid feeding insects digestion begin before the food is ingested through the injection or regurgitation of enzymes on to the food . In general it occurs in the mid gut (most of the enzymes are produced). The enzymes break down the complex substances in the food into simple substances which can be absorbed and assimilated. Enzymes function optimally only within limited range of pH and temperature. Absorption is passive process in some cases , but in others active transport occurs. Absorption of water is important in terrestrial insects , rectum remove water from faeces. • Insects of different groups consume astonishing variety of foods, including watery xylem sap, vertebrate blood, dry wood, bacteria and algae and the internal tissues of other insects. • Types of mouth parts correlates with the diets of insect. Also gut structure and function affected by nutrient composition of the food eaten by insect. • Insects that take solid food typically have a wide, straight, short gut with strong musculature and obvious protection from abrasion (especially in the midgut, which has no cuticular lining). • These features are most obvious in solid-feeders with rapid throughput of food, as in many insects and plant-feeding caterpillars In contrast, insects feeding on blood, sap or nectar usually have long, narrow, convoluted guts to allow maximal contact with the liquid food; here, protection from abrasion is unnecessary. The most obvious gut specialization of liquid-feeders is a mechanism for removing excess water to concentrate nutrient substances prior to digestion, as seen in hemipterans. -

A Combined Approach of Teaching Head Development Using Embryology and Comparative Anatomy
Edorium J Anat Embryo 2016;3:17–27. Danowitz et al. 17 www.edoriumjournals.com/ej/ae REVIEW ARTICLE PEER REVIEWED | OPEN ACCESS A combined approach of teaching head development using embryology and comparative anatomy Melinda Danowitz, Hong Zheng, Adriana Guigova, Nikos Solounias ABSTRACT the evolutionary changes of many structures allows for a greater understanding of the Many aspects of human head embryology reflect human embryology, and removes the need for its evolutionary development. The pharyngeal memorization of seemingly complex processes. arches, a major component of head development, A link to comparative evolutionary anatomy originally functioned in filter feeding and provides context to the purpose and morphology vascular exchange, which is why each arch of primitive structures, and clarifies several has associated vasculature and muscles. The issues in human head development. primitive tongue had few-associated muscles and was responsible for simple movements; the Keywords: Anatomy education, Embryology, Head human tongue evolved post-otic somites that and neck, Pharyngeal arches migrate to the tongue and develop the majority of the tongue musculature. These somites originate How to cite this article outside the tongue, and the motor innervation therefore differs from the general and special Danowitz M, Zheng H, Guigova A, Solounias N. A sensory innervation. In the primitive condition, combined approach of teaching head development the trapezius and sternocleidomastoid belonged using embryology and comparative anatomy. to a single muscle group that were involved in Edorium J Anat Embryo 2016;3:17–27. gill movements; they separate into two muscles with the reduction of certain skeletal elements, but retain the same innervation. -
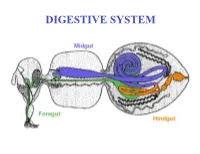
DIGESTIVE SYSTEM Generalized Insect Alimentary Tract the Digestive System Is Just a Tube Within a Surrounding Tube Called the Body
DIGESTIVE SYSTEM Generalized insect alimentary tract The digestive system is just a tube within a surrounding tube called the body. It starts with a mouth and ends with the anus. What goes on in between depends on the insect, its life stage and what it eats. The origin of the digestive tract. At the anterior pole of the embryo an indentation forms that will be the foregut or stomodeum. At the other end a similar thing occurs and the proctodeum or hindgut is formed. Both are lined by cuticle. They both are of ectodermal origin while the midgut is of mesodermal origin and is also called the mesenteron. This different origin of the midgut from the endoderm and not the ecotoderm probably explains why it is not lined with cuticle Anterior midgut invagination. In the bottom photo note the invagination starting forming the ventral furrow lumen (VF) MIDGUT FORMATION IN THE EMBRYO PMG in the above embryo shows the posterior midgut invagination cup where the posterior invagination shown in the drawing on the right will take place. Photo of Drosophila embryo. Hindgut invagination DIGESTIVE SYSTEM The digestive tract not only aids in obtaining, processing and digesting food molecules - It is the largest endocrine tissue in both humans and insects. The digestive system is involved in: 1. Obtaining food 2. Mechanically breaking it down into smaller particles that facilitate digestive enzymes acting on them 3. Enzymatic breakdown of larger food molecules into molecules that can pass through the digestive tract (midgut) and enter the hemolymph 4. Produces molecules or MESSENGERS (eg. -

Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects
Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects J.M. Johnson SUMMARY: A variety of congenital syndromes affecting the face occur due to defects involving the G. Moonis first and second BAs. Radiographic evaluation of craniofacial deformities is necessary to define aberrant anatomy, plan surgical procedures, and evaluate the effects of craniofacial growth and G.E. Green surgical reconstructions. High-resolution CT has proved vital in determining the nature and extent of R. Carmody these syndromes. The radiologic evaluation of syndromes of the first and second BAs should begin H.N. Burbank first by studying a series of isolated defects: CL with or without CP, micrognathia, and EAC atresia, which compose the major features of these syndromes and allow more specific diagnosis. After discussion of these defects and the associated embryology, we proceed to discuss the VCFS, PRS, ACS, TCS, Stickler syndrome, and HFM. ABBREVIATIONS: ACS ϭ auriculocondylar syndrome; BA ϭ branchial arch; CL ϭ cleft lip; CL/P ϭ cleft lip/palate; CP ϭ cleft palate; EAC ϭ external auditory canal; HFM ϭ hemifacial microsomia; MDCT ϭ multidetector CT; PRS ϭ Pierre Robin sequence; TCS ϭ Treacher Collins syndrome; VCFS ϭ velocardiofacial syndrome adiographic evaluation of craniofacial deformities is nec- major features of the syndromes of the first and second BAs. Ressary to define aberrant anatomy, plan surgical proce- Part 2 of this review discusses the syndromes and their radio- dures, and evaluate the effects of craniofacial growth and sur- graphic features: PRS, HFM, ACS, TCS, Stickler syndrome, gical reconstructions.1 The recent rapid proliferation of and VCFS. -

Branchial and Thyroglossal Cysts and Fistulae
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1942 Branchial and thyroglossal cysts and fistulae George G. Johnson University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Johnson, George G., "Branchial and thyroglossal cysts and fistulae" (1942). MD Theses. 929. https://digitalcommons.unmc.edu/mdtheses/929 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. BRANCHIAL & THYROGLOSSAL CYSTS & FISTULAE GEORGE N. JOHNSON SENIOR THESIS PRESENTED TO THE COLLEGE OF MEDICINE APRIL 6, 1942 INDEX BRANCHIAL CYSTS AND FISTULAE Page Introduction l Historical background 1 Embryological background 10 Wenglowski's embryology of the Branehia.l apparatus 14 Arey 1 s embryology of the Branchial apparatus 16 Symptoms 22 Age incidence 23 Diagnosis 24 Treatment 27 ------THYROGLOSSAL --DUCT CYSTS AND ----FISTULAE Historical background 32 Embryological history 34 Weller's embryology of the Thyroid 35 Arey's embryology of the Thyroid 39 Clinical features 40 Location 45 Age incidence 46 Pathology 47 Symptoms & Diagnosis 48 Treatment 51 481309 INTRODUCTION This thesis will be confined entirely to branch- ial and thyroglossal duct cysts and fistulas with the purpose of covering the two separate disease entities in one paper. It is also important to mention that because of the close similarity of these two patho- . -
![The Pharyngeal Arches [PDF]](https://docslib.b-cdn.net/cover/8257/the-pharyngeal-arches-pdf-1328257.webp)
The Pharyngeal Arches [PDF]
24.3.2015 The Pharyngeal Arches Dr. Archana Rani Associate Professor Department of Anatomy KGMU UP, Lucknow What is Pharyngeal Arch? • Rod-like thickenings of mesoderm present in the wall of the foregut. • They appear in 4th-5th weeks of development. • Contribute to the characteristic external appearance of the embryo. • As its development resembles with gills (branchia: Greek word) in fishes & amphibians, therefore also called as branchial arch. Formation of Pharyngeal Arches Lens N Pharyngeal Apparatus Pharyngeal apparatus consists of: • Pharyngeal arches • Pharyngeal pouches • Pharyngeal grooves/clefts • Pharyngeal membrane Pharyngeal Arches • Pharyngeal arches begin to develop early in the fourth week as neural crest cells migrate into the head and neck region. • The first pair of pharyngeal arches (primordium of jaws) appears as a surface elevations lateral to the developing pharynx. • Soon other arches appear as obliquely disposed, rounded ridges on each side of the future head and neck regions. Le N Pharyngeal Arches • By the end of the fourth week, four pairs of pharyngeal arches are visible externally. • The fifth and sixth arches are rudimentary and are not visible on the surface of the embryo. • The pharyngeal arches are separated from each other by fissures called pharyngeal grooves/clefts. • They are numbered in craniocaudal sequence. Pharyngeal Arch Components • Each pharyngeal arch consists of a core of mesenchyme. • Is covered externally by ectoderm and internally by endoderm. • In the third week, the original mesenchyme is derived from mesoderm. • During the fourth week, most of the mesenchyme is derived from neural crest cells that migrate into the pharyngeal arches. Structures in a Pharyngeal Arch Arrangement of nerves supplying the pharyngeal arch (in lower animals) Fate of Pharyngeal Arches A typical pharyngeal arch contains: • An aortic arch, an artery that arises from the truncus arteriosus of the primordial heart.