Functional Responses and Intraspecific
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lucan's Natural Questions: Landscape and Geography in the Bellum Civile Laura Zientek a Dissertation Submitted in Partial Fulf
Lucan’s Natural Questions: Landscape and Geography in the Bellum Civile Laura Zientek A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2014 Reading Committee: Catherine Connors, Chair Alain Gowing Stephen Hinds Program Authorized to Offer Degree: Classics © Copyright 2014 Laura Zientek University of Washington Abstract Lucan’s Natural Questions: Landscape and Geography in the Bellum Civile Laura Zientek Chair of the Supervisory Committee: Professor Catherine Connors Department of Classics This dissertation is an analysis of the role of landscape and the natural world in Lucan’s Bellum Civile. I investigate digressions and excurses on mountains, rivers, and certain myths associated aetiologically with the land, and demonstrate how Stoic physics and cosmology – in particular the concepts of cosmic (dis)order, collapse, and conflagration – play a role in the way Lucan writes about the landscape in the context of a civil war poem. Building on previous analyses of the Bellum Civile that provide background on its literary context (Ahl, 1976), on Lucan’s poetic technique (Masters, 1992), and on landscape in Roman literature (Spencer, 2010), I approach Lucan’s depiction of the natural world by focusing on the mutual effect of humanity and landscape on each other. Thus, hardships posed by the land against characters like Caesar and Cato, gloomy and threatening atmospheres, and dangerous or unusual weather phenomena all have places in my study. I also explore how Lucan’s landscapes engage with the tropes of the locus amoenus or horridus (Schiesaro, 2006) and elements of the sublime (Day, 2013). -

Synthetic Worlds Nature, Art and the Chemical Industry
Synthetic Worlds Nature, Art and the Chemical Industry Esther Leslie Synthetic Worlds Synthetic Worlds Nature, Art and the Chemical Industry Esther Leslie reaktion books Published by reaktion books ltd www.reaktionbooks.co.uk First published 2005 Copyright © Esther Leslie 2005 All rights reserved No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers. Colour printed by Creative Print and Design Group, Harmondsworth, Middlesex Printed and bound in Great Britain by Biddles Ltd, Kings Lynn British Library Cataloguing in Publication Data Leslie, Esther, 1964– Synthetic worlds: nature, art and the chemical industry 1.Art and science 2.Chemical industry - Social aspects 3.Nature (Aesthetics) I. Title 7-1'.05 isbn 1 86189 248 9 Contents introduction: Glints, Facets and Essence 7 one Substance and Philosophy, Coal and Poetry 25 two Eyelike Blots and Synthetic Colour 48 three Shimmer and Shine, Waste and Effort in the Exchange Economy 79 four Twinkle and Extra-terrestriality: A Utopian Interlude 95 five Class Struggle in Colour 118 six Nazi Rainbows 167 seven Abstraction and Extraction in the Third Reich 193 eight After Germany: Pollutants, Aura and Colours That Glow 218 conclusion: Nature’s Beautiful Corpse 248 References 254 Select Bibliography 270 Acknowledgements 274 Index 275 introduction Glints, Facets and Essence opposites and origins In Thomas Pynchon’s novel Gravity’s Rainbow a character remarks on an exploding missile whose approaching noise is heard only afterwards. The horror that the rocket induces is not just terror at its destructive power, but is a result of its reversal of the natural order of things. -
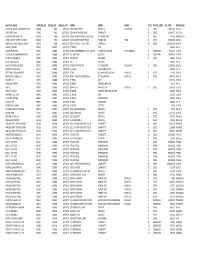
Supplier Name
SUPPLIER_NAME SUPPLIER_NO CHEQUE_NO CHEQUE_DATE ADDR1 ADDR2 ADDR3 STATE POSTAL_CODE INV_PAID INVOICE_NO OFFICE OF LOCAL GOVERNMENT SRF 04368 9683 6/3/2019 1201 MAIN STREET SUITE 910 COLUMBIA SC 29201 13,825.00 5-31-19 SOUTHEAST JACK C4690 9684 6/17/2019 7000 W WT HARRIS BLVD CHARLOTTE NC 28269 14,064.73 L6-17-19 GLOBAL AEROSPACE, INC. 09568 9686 6/17/2019 10895 GRANDVIEW DR, SUITE 150 OVERLAND PARK KS 66210 975.00 I00061547/01 YORK COUNTY CLERK OF COURT 00538 9687 6/26/2019 YORK COUNTY COURTHOUSE P O BOX 649 YORK SC 29745 35,000.00 6-26-19 JAMES, MCELROY & DIEHL TRUST 09567 9688 6/26/2019 525 N TRYON ST., SUITE 700 CHARLOTTE NC 28202 125,000.00 6-26-19 GIBSON, STEVEN 00957 230879 6/7/2019 EE #2679 OMB 209.96 6-2-19 UNUM PROVIDENT 01935 230880 6/7/2019 ATTN: VWB PREMIUM CLTN. & ACCT 1 FOUNTAIN SQUARE CHATTANOOGA TN 374021362 3,742.76 4-25-19 COMPORIUM COMMUNICATIONS 01951 230881 6/7/2019 P.O. BOX 1042 ROCK HILL SC 297317042 14,949.05 5-29-19 SC DEPT OF REVENUE 02000 230882 6/7/2019 TAX RETURN COLUMBIA SC 29214 486.00 5-31-19 SCOTT, MICHAEL W. 03826 230883 6/7/2019 CRH FIRE DEPT 200.00 6-5-19 BLUE CROSS BLUE SHIELD 04295 230884 6/7/2019 OF SOUTH CAROLINA P.O. BOX 6000 COLUMBIA SC 29260 11,959.40 5-21-19 BROWN, PAULA KNOX 06154 230885 6/7/2019 EE # 5036 SOLICITOR'S OFFICE 100.00 6-5-19 PRT TENNIS TOURNAMENT 06465 230886 6/7/2019 ATTN: 897 MAPLEWOOD LANE ROCK HILL SC 29730 112.00 5-29-19 NEXT LEVEL TENNIS, LLC 06525 230887 6/7/2019 ATTN: TEODORA DONCHEVA 872 DILLARD RD ROCK HILL SC 29730 7,897.60 N6-3-19 BROWN, LISA 07325 230888 6/7/2019 EE #5855 OMB 1,673.20 5-29-19 WINKLER, JEREMY 07922 230889 6/7/2019 EE #4207 GENERAL SERVICES 81.20 6-3-19 H.O.P.E. -

Numerical Response of Olla V-Nigrum (Coleoptera: Coccinellidae) to Infestations of Asian Citrus Psyllid, (Hemiptera: Psyllidae) in Florida
608 Florida Entomologist 84(4) December 2001 NUMERICAL RESPONSE OF OLLA V-NIGRUM (COLEOPTERA: COCCINELLIDAE) TO INFESTATIONS OF ASIAN CITRUS PSYLLID, (HEMIPTERA: PSYLLIDAE) IN FLORIDA J. P. MICHAUD University of Florida, Citrus Research and Education Center, 700 Experiment Station Road, Lake Alfred, FL 33881 ABSTRACT Data are presented on the relative abundance of the coccinellid Olla v-nigrum (Mulsant) in Florida citrus, before and after invasion by the Asian citrus psyllid, Diaphorina citri Ku- wayama. Adults and larvae of O. v-nigrum were observed preying on immature psyllids throughout their range in Florida. Immature psyllids were eliminated by predation from many flushed citrus terminals that exhibited damage symptoms; pupae of O. v-nigrum and Harmonia axyridis Pallas were recovered from adjacent leaves. Olla v-nigrum, a relatively rare species before the invasion by D. citri, is now a dominant species throughout Florida in citrus groves where the psyllid is present, but remains rare in regions where D. citri is ab- sent. The strong numerical response of this native ladybeetle to D. citri populations indi- cates that it is assuming a key role in biological control of the psyllid. Key Words: abundance, biological control, coccinellids, Diaphorina citri, Harmonia axyridis, Olla v-nigrum RESUMEN Se presentan datos sobre la abundancia relativa del coccinélido Olla v-nigrum (Mulsant) en cítricos en la Florida, antes y después de la invasión del psílido Asiático, Diphorina citri Kuwayama. Adultos y larvas de Olla v-nigrum fueron observados alimentándose de las for- mas inmaduras del psílido a través de la Florida. Se observaron muchos brotes terminales en los cítrcos con daños del psílido, pero estos fueron eliminados por depredación; pupas de O. -

Greek Mythology Link (Complete Collection)
Document belonging to the Greek Mythology Link, a web site created by Carlos Parada, author of Genealogical Guide to Greek Mythology Characters • Places • Topics • Images • Bibliography • Español • PDF Editions About • Copyright © 1997 Carlos Parada and Maicar Förlag. This PDF contains portions of the Greek Mythology Link COMPLETE COLLECTION, version 0906. In this sample most links will not work. THE COMPLETE GREEK MYTHOLOGY LINK COLLECTION (digital edition) includes: 1. Two fully linked, bookmarked, and easy to print PDF files (1809 A4 pages), including: a. The full version of the Genealogical Guide (not on line) and every page-numbered docu- ment detailed in the Contents. b. 119 Charts (genealogical and contextual) and 5 Maps. 2. Thousands of images organized in albums are included in this package. The contents of this sample is copyright © 1997 Carlos Parada and Maicar Förlag. To buy this collection, visit Editions. Greek Mythology Link Contents The Greek Mythology Link is a collection of myths retold by Carlos Parada, author of Genealogical Guide to Greek Mythology, published in 1993 (available at Amazon). The mythical accounts are based exclusively on ancient sources. Address: www.maicar.com About, Email. Copyright © 1997 Carlos Parada and Maicar Förlag. ISBN 978-91-976473-9-7 Contents VIII Divinities 1476 Major Divinities 1477 Page Immortals 1480 I Abbreviations 2 Other deities 1486 II Dictionaries 4 IX Miscellanea Genealogical Guide (6520 entries) 5 Three Main Ancestors 1489 Geographical Reference (1184) 500 Robe & Necklace of -

LUKE A. NICHTER Professor of History Texas A&M University
Curriculum Vitae Last Updated: August 22, 2018 LUKE A. NICHTER Professor of History Texas A&M University – Central Texas Department of Humanities 217 Founder’s Hall 1001 Leadership Place Killeen, Texas 76549 Phone: +1 (254) 519-5735 Email: [email protected] Web: lukenichter.com PROFESSIONAL BIO Luke A. Nichter is Professor of History at Texas A&M University – Central Texas, Book Review Editor for Presidential Studies Quarterly, Visiting Research Fellow at Bowling Green State University, and National Endowment for the Humanities Public Scholar. He has been Visiting Fellow at the Norwegian Nobel Institute, Andrew W. Mellon Fellow at the Massachusetts Historical Society, Visiting Scholar at the University of Michigan’s Eisenberg Institute for Historical Studies, Senior Visiting Research Fellow at the University of Oxford’s Rothermere American Institute, and Hansard Research Scholar at the London School of Economics. Luke is a noted expert on Richard Nixon’s 3,451 hours of secret White House tapes. He is a New York Times bestselling author or editor of six books, including Richard Nixon and Europe: The Reshaping of the Postwar Atlantic World (Cambridge University Press), which was based on multilingual archival research in six countries. His current book project is Henry Cabot Lodge, Jr. and the Decline of the Eastern Establishment, to be published by Yale University Press. It will be the first full biography of Lodge – whose public career spanned from the 1930s to the 1970s – also based on extensive multilingual archival research. Also, Luke is the author, with Douglas Brinkley, of The Nixon Tapes: 1971-1972 (Houghton Mifflin Harcourt), with a Mandarin version to appear in the near future by the premier Chinese academic publisher SDX (Sanlian) Joint Publishing Company. -

HEALTHCARE BUSINESS MONTHLY July 2016 Coding | Billing | Auditing | Compliance | Practice Management
HEALTHCARE BUSINESS MONTHLY July 2016 www.aapc.com Coding | Billing | Auditing | Compliance | Practice Management Get Paid for Smoking Cessation: 22 Don’t give up on reimbursement: Verify coverage Exude Confidence as an Auditor: 52 Ditch the emotional baggage and gain respect Tips to Improve HEDIS Scores: 60 Enhance quality of care and reduce costs Smart Design. Intelligent Auditing. Healthicity - 1 Customize, manage, train and simplify your audit process. We streamlined your audit process by merging audit workflow, management, and reporting capabilities into one easy-to-use, web-based solution. HEALTHICITY.COM/AUDITMANAGER Healthcare Business Monthly | July 2016 COVER | Coding/Billing | 38 Secrets of Successful Coders Apply 14 strategies to help you climb your way to coding success. Smart Design. By Stephanie Cecchini, CPC, CEMC, CHISP Intelligent Auditing. Healthicity - 1 [contents] ■ Coding/Billing ■ Added Edge ■ Practice Management Customize, manage, train and simplify your audit process. We streamlined your audit process by merging audit workflow, management, and reporting 22 Get Paid for Smoking Cessation 52 Ditch the Emotional Baggage to 56 Manage Hospital capabilities into one easy-to-use, web-based solution. Kasandra Bolzenius, CPC Become a Respected Auditor Staff Cellphone Distractions Holly Pettigrew, COC, CPC, CHC Michelle A. Dick HEALTHICITY.COM/AUDITMANAGER [continued on next page] www.aapc.com July 2016 3 Healthcare Business Monthly | July 2016 | contents 16 ■ Member Feature 14 Emory Physician Group Practice Celebrates and Prepares Its Coders Donna Beaulieu, CRC, C-CDIS, CPC-I, CPMA, CPC, CEMC, CEDC, CFPC, CCP-P, CRP Hasan Zaidi, MPH, CPC, CEDC, CSPPM ■ Coding/Billing 16 MACRA FAQs Renee Dustman 20 Think Twice Before Sticking It in Your Ear Maryann C. -

Abhiyoga Jain Gods
A babylonian goddess of the moon A-a mesopotamian sun goddess A’as hittite god of wisdom Aabit egyptian goddess of song Aakuluujjusi inuit creator goddess Aasith egyptian goddess of the hunt Aataentsic iriquois goddess Aatxe basque bull god Ab Kin Xoc mayan god of war Aba Khatun Baikal siberian goddess of the sea Abaangui guarani god Abaasy yakut underworld gods Abandinus romano-celtic god Abarta irish god Abeguwo melansian rain goddess Abellio gallic tree god Abeona roman goddess of passage Abere melanisian goddess of evil Abgal arabian god Abhijit hindu goddess of fortune Abhijnaraja tibetan physician god Abhimukhi buddhist goddess Abhiyoga jain gods Abonba romano-celtic forest goddess Abonsam west african malicious god Abora polynesian supreme god Abowie west african god Abu sumerian vegetation god Abuk dinkan goddess of women and gardens Abundantia roman fertility goddess Anzu mesopotamian god of deep water Ac Yanto mayan god of white men Acacila peruvian weather god Acala buddhist goddess Acan mayan god of wine Acat mayan god of tattoo artists Acaviser etruscan goddess Acca Larentia roman mother goddess Acchupta jain goddess of learning Accasbel irish god of wine Acco greek goddess of evil Achiyalatopa zuni monster god Acolmitztli aztec god of the underworld Acolnahuacatl aztec god of the underworld Adad mesopotamian weather god Adamas gnostic christian creator god Adekagagwaa iroquois god Adeona roman goddess of passage Adhimukticarya buddhist goddess Adhimuktivasita buddhist goddess Adibuddha buddhist god Adidharma buddhist goddess -

Communication and Legal Expression in Performance Cultures
Pittsburgh University School of Law Scholarship@PITT LAW Articles Faculty Publications 1992 'Coming to Our Senses': Communication and Legal Expression in Performance Cultures Bernard J. Hibbitts University of Pittsburgh School of Law, [email protected] Follow this and additional works at: https://scholarship.law.pitt.edu/fac_articles Part of the Indian and Aboriginal Law Commons, Law and Philosophy Commons, Law and Psychology Commons, Law and Society Commons, and the Other Law Commons Recommended Citation Bernard J. Hibbitts, 'Coming to Our Senses': Communication and Legal Expression in Performance Cultures, 41 Emory Law Journal 41 873 (1992). Available at: https://scholarship.law.pitt.edu/fac_articles/123 This Article is brought to you for free and open access by the Faculty Publications at Scholarship@PITT LAW. It has been accepted for inclusion in Articles by an authorized administrator of Scholarship@PITT LAW. For more information, please contact [email protected], [email protected]. EMORY LAW JOURNAL Volume 41 FALL 1992 Number 4 ARTICLES "COMING TO OUR SENSES": COMMUNICATION AND LEGAL EXPRESSION IN PERFORMANCE CULTURES BernardJ. Hibbitts* Table of Contents I. INTRODUCTION ...................................... 874 II. COMMUNICATION AND LEGAL EXPRESSION IN PERFORMANCE CULTURES: A TENTATIVE TYPOLOGY... 888 A. Aural Communication and Legal Expression ..... 889 1. The Cultural Significance of Sound ........ 889 2. The Sound of Law ....................... 895 B. Visual Communication and Legal Expression ..... 905 1. The Cultural Significance of Sight .......... 905 2. The Sight of Law ........................ 910 C. Tactile Communication and Legal Expression .... 924 1. The Cultural Significance of Touch ......... 924 2. The Feel of Law ......................... 927 * Associate Professor, University of Pittsburgh School of Law. Special thanks must go to Charles Donahue, Harr-y Flechtner, and several anonymous tenure evaluators for reviewing either drafts of this Article or the manuscript from which it is drawn. -

Butterflies of India Paul Van
Butterflies of India – Annotated Checklist Compiled By Paul Van Gasse Family Hesperiidae Subfamily Coeliadinae • Burara oedipodea (Branded Orange Awlet) B.o.ataphus: Sri Lanka. NR – Ceylon 17 B.o.belesis: Kangra to Arunachal, NE India, and Burma to Dawnas (= aegina, athena) – NW Himalayas (Kangra-Kumaon) 11, Sikkim 30, Bhutan 2, Assam 28, Burma (to Dawnas) 9 B.o.oedipodea: Probably S Burma. [Given as Ismene oedipodea in Evans, 1932, and as Bibasis oedipodea in Evans, 1949] • Burara tuckeri (Tucker’s Awlet) Burma in Tavoy. VR – Tavoy 1 [Given as Ismene tuckeri in Evans, 1932, and as Bibasis tuckeri in Evans, 1949] • Burara jaina (Orange Awlet) B.j.fergusonii: SW India to N Maharashtra. NR – S India 33 B.j.jaina: HP (Solan) and Garhwal to Arunachal, NE India, and Burma to Karens. NR (= vasundhara) – NW Himalayas (Dun-Kumaon) 3, Sikkim 18, Assam 37, Burma (Karens) 1 B.j.margana: Burma in Dawnas. R – Burma (Dawnas) 8 B.j.astigmata: S Andamans. VR – Andamans 3 [Given as Ismene jaina in Evans, 1932, and vasundhara was there given as the subspecies ranging from Assam to Karens, with jaina then confined to Mussoorie to Sikkim; given as Bibasis jaina in Evans, 1949] • Burara anadi (Plain Orange Awlet) Garhwal to NE India and Burma to Karens. R (= purpurea) – Mussoorie 1, Sikkim 13, Assam 1, Burma (Karens) 5 [Given as Ismene anadi in Evans, 1932, and as Bibasis anadi in Evans, 1949] • Burara etelka (Great Orange Awlet) NE India (Kabaw Valley in Manipur). Burma from Karens S. R – Karens to Mergui 14 [Given as Ismene etelka in Evans, 1932, and as Bibasis etelka in Evans, 1949] • Burara harisa (Orange Awlet) B.h.harisa: C Nepal to NE India and Burma. -

GRŠKE MITOLOŠKE OSEBE 1. Del – 1 IMMORTALS
BIOtransfer GRŠKE MITOLOŠKE OSEBE 1. del – 1 IMMORTALS GRŠKE MITOLOŠKE OSEBE 1. del IMMORTALS KAZALO: 1 Immortals 1.1 Olympian deities 2 1.2 Protogenoi (primordial) 3 1.3 Titans 4 1.4 Gigantes (giants) 6 1.5 Personified concepts 7 1.6 Chthonic deities 12 1.7 Sea deities 13 1.8 Sky deities 16 1.9 Rustic deities 19 1.10 Agricultural deities 31 1.11 Deified mortals 32 1.12 Health deities 33 1.13 Other deities 34 1.14 Seznam z merilnimi podatki o BIOtransferu 37 Vir: http://en.wikipedia.org/wiki/Greek_gods Ljubljana, 04.04.2013 Stran 1 od 50 121010_BR_FOTO_grške_mitološke_osebe_1.del BIOtransfer GRŠKE MITOLOŠKE OSEBE 1. del – 1 IMMORTALS 1 IMMORTALS 1.1 OLYMPIAN DEITIES TWELVE OLYMPIANS 1. Aphrodite 2. Apollo 3. Ares 4. Artemis 5. Athena 6. Demeter 7. Dionysus 8. Hades 9. Hephaestus 10. Hera 11. Hermes 12. Hestia 13. Poseidon 14. Zeus Ljubljana, 04.04.2013 Stran 2 od 50 121010_BR_FOTO_grške_mitološke_osebe_1.del BIOtransfer GRŠKE MITOLOŠKE OSEBE 1. del – 1 IMMORTALS 1.2 PROTOGENOI (PRIMORDIAL) 15. Aether 16. Ananke 17. Erebos or Erebus 18. Gaia or Gaea 19. Hemera 20. Chaos 21. Chronos 22. The Nesoi 23. Nyx or Night 24. Uranus 25. The Ourea 26. Phanes 27. Pontus 28. Tartarus 29. Thalassa Ljubljana, 04.04.2013 Stran 3 od 50 121010_BR_FOTO_grške_mitološke_osebe_1.del BIOtransfer GRŠKE MITOLOŠKE OSEBE 1. del – 1 IMMORTALS 1.3 TITANS THE TWELVE TITANS 30. Hyperion 31. Iapetus 32. Coeus 33. Crius 34. Cronus 35. Mnemosyne 36. Oceanus 37. Phoebe 38. Rhea 39. Tethys 40. Theia 41. Themis Ljubljana, 04.04.2013 Stran 4 od 50 121010_BR_FOTO_grške_mitološke_osebe_1.del BIOtransfer GRŠKE MITOLOŠKE OSEBE 1. -

Plague and the End of Antiquity : the Pandemic of 541-750
P1: JZP 0521846390pre CUFX041/Little 0521 84639 0 printer: cupusbw October 20, 2006 10:47 This page intentionally left blank ii P1: JZP 0521846390pre CUFX041/Little 0521 84639 0 printer: cupusbw October 20, 2006 10:47 Plague and the End of Antiquity Plague was a key factor in the waning of Antiquity and the beginning of the Middle Ages. Eight centuries before the Black Death, a pan- demic of plague engulfed the lands surrounding the Mediterranean Sea and eventually extended as far east as Persia and as far north as the British Isles. It persisted sporadically from 541 to 750, the same period that witnessed the distinctive shaping of the Byzantine Empire, a new prominence of the Roman papacy and of monasticism, the begin- nings of Islam and the meteoric expansion of the Arabic Empire, the ascent of the Carolingian dynasty in Frankish Gaul, and, not coinci- dentally, the beginnings of a positive work ethic in the Latin West. In this volume, twelve scholars using history, archaeology, epidemiol- ogy, and molecular biology have produced a comprehensive account of the pandemic’s origins, spread, and mortality, as well as its eco- nomic, social, political, and religious effects. The historians’ sources are in Arabic, Syriac, Greek, Latin, and Old Irish. The archaeologists’ sources include burial pits, abandoned villages, and aborted build- ing projects. The epidemiologists use the written sources to track the disease’s means and speed of transmission, the mix of vulnerability and resistance it encountered, and the patterns of reappearance over time. Finally, molecular biologists, newcomers to this kind of inves- tigation, have become pioneers of paleopathology, seeking ways to identify pathogens in human remains from the remote past.