Meeting Agenda
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St
2014;22(1):13-18 TCP Translational and Clinical Pharmacology http://dx.doi.org/10.12793/tcp.2014.22.1.13 Clinical Pharmacology Review for Primary Health Care Providers: I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul 137-701, Korea *Correspondence: S. Han; Tel: +82-2-2258-7326, Fax: +82-2-2258-7876, E-mail: [email protected] Received 31 May 2014 Primary health care providers play a critical role in maintaining public health, and the appropri- Accepted 31 May 2014 ate use of pharmaceutical products is one of the major parts of their practice. This series of articles, pISSN: 2289-0882 entitled ‘Clinical Pharmacology Review for Primary Health Care Providers,’ is intended to help pri- mary health care providers select more appropriate prescriptions for frequently used drugs based on up-to-date information. We expect that this effort will contribute to improvements in public health and diminish unnecessary drug use. Introduction tion on the H1 receptor.[8] THERAPEU Antihistamines include some of the most frequently prescribed drugs in the primary health care environment for the symp- Generations and Classes tomatic relief of allergic diseases, the common cold, urticaria, Many primary health care providers are well-informed about T and insomnia.[1-5] The importance of antihistamines has been the different ‘generations’ of antihistamines but not about the ICS TU emphasized as the prevalence of target diseases increases.[6,7] different ‘classes’ characterized according to chemical structure. However, the appropriate use, clinical effectiveness, and target [1] This discrepancy seems reasonable because ‘inter-generation’ T populations for prescription of antihistamines are still a matter differences are more prominent than ‘inter-class’ differences. -

FDA Warns That Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm This Includes Heart and Mental Health Problems Or Death
FDA warns that abuse and misuse of the nasal decongestant propylhexedrine causes serious harm This includes heart and mental health problems or death 3-25-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that the abuse and misuse of the over- the-counter (OTC) nasal decongestant propylhexedrine can lead to serious harm such as heart and mental health problems. Some of these complications, which include fast or abnormal heart rhythm, high blood pressure, and paranoia, can lead to hospitalization, disability, or death. Reports of individuals abusing and misusing propylhexedrine have increased in recent years. Propylhexedrine is safe and effective when used as directed. What is FDA doing? We are requesting that all manufacturers of OTC propylhexedrine nasal decongestant inhalers consider product design changes that support its safe use. For example, modifying the product to create a physical barrier that would make tampering with the device and abusing the propylhexedrine inside more difficult. In addition, decreasing the amount of medicine the device contains could also reduce the risk of serious side effects if abused or misused. We continue to evaluate this safety issue and will determine if additional FDA actions are needed. What is propylhexedrine and how can it help me? Propylhexedrine is a nasal decongestant that is available OTC in an inhaler. It is used short term to temporarily relieve nasal congestion due to colds, hay fever, or other upper respiratory allergies. It works by reducing swelling and inflammation of the mucous membrane lining of the nose. -
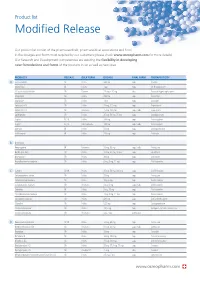
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Sudafed Decongestant Tablets 12S PIL SNAS 3359 SNAS 3557 14 Mm 29.07.2021 English Rate and Blood Pressure)
12 mm 12 mm spot = 12 x 2 centered in Distance between cutted the margin of 14 mm line/middel of the spot = 51.6 mm UK_Sudafed Decongestant Tablets 12s_PIL_SNAS 3359_SNAS 3557 14 mm 29.07.2021 English rate and blood pressure). 402059927_r2 N/A Now read this whole leaflet carefully before you use this medicine. Keep the leaflet: you ■ If you have an overactive thyroid Brochure/Leaet/Insert 145 x 250 mm might need it again. gland. 360165B BL-Val de Reuil_France ■ If you have glaucoma (increased CHILD 360165A Multi Packaging Solutions St Pierre 1 What the medicine is for pressure in the eye). 953 Oset Printing Sudafed Decongestant Tablets is a medicine used ■ If you have severe kidney problems. 8169503 PAPER to provide relief from cold, flu and allergy ■ If you are taking beta blockers (used to EMEA_2020_00006767_001 PC-0002778 symptoms such as blocked sinuses, stuffy nose treat high blood pressure). and catarrh. ■ If you are taking, or have taken in the The tablets contain pseudoephedrine last two weeks, drugs for depression ■ This medicine is used to help clear cold, flu hydrochloride, which is a decongestant that known as Monoamine Oxidase and allergy symptoms such as blocked relieves nasal and sinus congestion. Inhibitors (MAOIs) or Reversible Black PANTONE sinuses, stuffy nose and catarrh. This medicine is for use by adults and children Inhibitors of Monoamine Oxidase 3005 C ■ This medicine is for use by adults and (RIMAs). LITHO OFFSET LITHO OFFSET aged 12 years and over. children aged 12 years and over. 145 mm REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval. -

Histamine and Antihistamines Sites of Action Conditions Which Cause Release Aron H
Learning Objectives I Histamine Pharmacological effects Histamine and Antihistamines Sites of action Conditions which cause release Aron H. Lichtman, Ph.D. Diagnostic uses Associate Professor II Antihistamines acting at the H1 and H2 receptor Pharmacology and Toxicology Pharmacological effects Mechanisms of action Therapeutic uses Side effects and drug interactions Be familiar with the existence of the H3 receptor III Be able to describe the main mechanism of action of cromolyn sodium and its clinical uses Histamine Pharmacology First autacoid to be discovered. (Greek: autos=self; Histamine Formation akos=cure) Synthesized in 1907 Synthesized in mammalian tissues by Demonstrated to be a natural constituent of decarboxylation of the amino acid l-histidine mammalian tissues (1927) Involved in inflammatory and anaphylactic reactions. Local application causes swelling redness, and edema, mimicking a mild inflammatory reaction. Large systemic doses leads to profound vascular changes similar to those seen after shock or anaphylactic origin Histamine Stored in complex with: Heparin Chondroitin Sulfate Eosinophilic Chemotactic Factor Neutrophilic Chemotactic Factor Proteases 1 Conditions That Release Histamine 1. Tissue injury: Any physical or chemical agent that injures tissue, skin or mucosa are particularly sensitive to injury and will cause the immediate release of histamine from mast cells. 2. Allergic reactions: exposure of an antigen to a previously sensitized (exposed) subject can immediately trigger allergic reactions. If sensitized by IgE antibodies attached to their surface membranes will degranulate when exposed to the appropriate antigen and release histamine, ATP and other mediators. 3. Drugs and other foreign compounds: morphine, dextran, antimalarial drugs, dyes, antibiotic bases, alkaloids, amides, quaternary ammonium compounds, enzymes (phospholipase C). -

Mucolytic Agents & Decongestants
Mucolytic Agents & Decongestants Mucolytic agents (Guaifenesin Mucinex® Humibid LA®) are drugs that thin mucus and secretions, so they can drain out of the sinuses more easily. They may be helpful for people suffering from a thick post-nasal drip. Often, they are found in combination preparations with decongestants and/or antihistamines. Most are well tolerated and have few side effects such as headache, rash, or nausea. Topical Nasal Decongestants Topical nasal decongestants (Afrin® or Neosynephrine®) in the form of drops or sprays that are placed in the nose can be very effective in shrinking nasal swelling in minutes. However, these sprays should be used no longer than 2 or 3 consecutive days, for prolonged usage may result in "rebound" swelling of the nose. Rebound swelling (known as "rhinitis medicamentosa") can be extremely difficult to treat because the nose may no longer respond normally to the decongestant spray. We advise that use the brands that contain oxymetazoline in preference to those that have phenylephrine as their active ingredient. The safe use of these medications sometimes can be extended by using the medicine only at bedtime, in alternating nostrils every other night. For example, on the first night only the right nostril receives medicine and on the second night the medicine is placed in your left nostril. By doing this some patients can double the safe use of oxymetazoline. Systemic Decongestants If more prolonged decongestant therapy is required, systemic decongestants may be used. The most well know brand is Sudafed®. It comes in varying strengths and is purchased over the counter. Psuedoephedrine can befound as part of a combination medication with some antihistamines such as Allegra® or Claritin®. -

Adverse Cardiac Effects of Decongestant Agents
Review Eur J Gen Med 2013; 10 (Suppl 1):32-35 Adverse Cardiac Effects Of Decongestant Agents Zeynettin Kaya, Abdullah Tuncez ABSTRACT Especially in spring and winter months many people use excessive dose topical or systemic decongestant agents to relief the al- lergic or infectious nasal congestive symptoms without physicians’ prescription. Although these agents bring some symptomatic benefits, sometimes serious adverse cardiac events reported at all drug users, especially who had known cardiac disease. These agents may cause slightly elevation of blood pressure in hypertensive patients, can trigger mortal arrhythmias like ventricular tachycardia, can also trigger myocardial infarction patients with known coronary artery disease or normal coronary artery and may cause decompensation of heart failure. In conclusion all these decongestant agents give only symptomatic relief and they don’t treat flu and allergic disease. For this reason if the symptoms are mild or moderate not using these agents will be more wisely. Key words: Decongestants, pseudoephedrine, phenylephrine, cardiac, arrhythmias Dekonjestan Ajanların Kardiyak Yan Etkileri ÖZET Özellikle bahar ve kış aylarında insanların birçoğu allerjik ya da enfeksiyona bağlı nasal konjesyonu gidermek için gereğinden fazla ve sıklıkla reçetesiz topikal veya sistemik dekonjestanları kullanmaktadırlar. Bu ilaçların semptomatik faydalarına karşın özellikle bilinen kardiyak hastalığı olanlar başta olmak üzere tüm kullanan kişilerde ciddi istenmeyen kardiyak olaylara neden olabildiği bildirilmiştir. Hipertansif hastalarda az da olsa kan basıncını yükselttiği, bazıları ventriküler taşikardi gibi ölümcül ola- bilen pek çok aritmiyi tetiklediği, bilinen koroner arter hastalığı olan ya da normal koroner anatomiye sahip kişilerde miyokard enfarktüsünü tetikleyebildiği, kalp yetmezliği olan hastalarda dekompansasyona neden olabildiği rapor edilmiştir. Sonuç olarak tüm dekonjestan ajanların allerjiyi, soğuk algınlığını tedavi etmediği sadece semptomatik rahatlama sağladığı unutulmamalıdır. -

Beechams Cold and Flu Capsules
• if you are taking tricyclic antidepressants V6 (e.g. imipramine or amitriptyline) or if you 04 - FEB - 2021 are taking or have taken within the last two weeks monoamine oxidase inhibitors (MAOIs) (e.g. moclobemide) prescribed for Artwork Information Panel depression D2A SGS No: COLD & FLU • if you are taking beta blockers (e.g. atenolol). 6069879 CAPSULES Do not take anything else containing Non Production Paracetamol, Phenylephrine, paracetamol while taking this medicine. CHAMPS No: Caffeine Do not take with any other flu, cold or Production X 0000001485 decongestant products. Manufacturing Site: Site Component No: Please read right through this leaflet before Take special care with Beechams Alcala 62000000051687 you start using this medicine. This medicine Cold & Flu Capsules is available without prescription, but you still • Contains paracetamol. Taking too much Market: Site Change Control No:Approving need to use Beechams Cold & Flu Capsules paracetamol can cause serious harm to Great Britain N/A carefully to get the best results from it. your liver. Product Market Trade Name: Component Type: • Keep this leaflet you may need to read it again. • This product may cause dizziness. If affected BEECHAMS_COLD & FLU Leaflet • If you have any questions, or if there is do not drive or operate machinery. anything you do not understand, ask your Technical Drawing No: Material Spec No: pharmacist. Ask your doctor before you L607D008 N/A take this medicine: In this leaflet: Print Process: Pharma Code No: • if you have a blood vessel disease such as Offset - Lithography 2339 1. What Beechams Cold & Flu Capsules do Raynaud’s Phenomenon 2. -

UQ272429 OA.Pdf
Cochrane Database of Systematic Reviews Oral antihistamine-decongestant-analgesic combinations for the common cold (Review) De Sutter AIM, van Driel ML, Kumar AA, Lesslar O, Skrt A De Sutter AIM, van Driel ML, Kumar AA, Lesslar O, Skrt A. Oral antihistamine-decongestant-analgesic combinations for the common cold. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No.: CD004976. DOI: 10.1002/14651858.CD004976.pub3. www.cochranelibrary.com Oral antihistamine-decongestant-analgesic combinations for the common cold (Review) Copyright © 2012 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 3 OBJECTIVES ..................................... 3 METHODS ...................................... 4 RESULTS....................................... 5 Figure1. ..................................... 7 Figure2. ..................................... 8 DISCUSSION ..................................... 19 AUTHORS’CONCLUSIONS . 20 ACKNOWLEDGEMENTS . 20 REFERENCES ..................................... 21 CHARACTERISTICSOFSTUDIES . 24 DATAANDANALYSES. 64 Analysis 1.1. Comparison 1 Combination 1: Antihistamine-decongestant, Outcome 1 Global evaluation. 66 Analysis 1.2. Comparison 1 Combination 1: Antihistamine-decongestant, Outcome 2 Side effects: all - Combination 1: Antihistamine-decongestant. 67 Analysis 1.3. Comparison 1 Combination 1: Antihistamine-decongestant, -
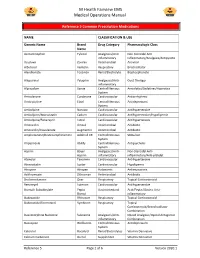
Reference 5 Common Prescription Medications
M Health Fairview EMS Medical Operations Manual Reference 5 Common Prescription Medications NAME CLASSIFICATION & USE Generic Name Brand Drug Category Pharmacologic Class Name Acetaminophen Tylenol Analgesics/Anti- Non-Steroidal Anti- inflammatory inflammatory/Analgesic/Antipyretic Acyclovir Zovirax Antimicrobial Antiviral Albuterol Ventolin Respiratory Brochodilator Alendronate Fosamax Renal/Electrolyte Bisphosphonate Allopurinol Zyloprim Analgesics/Anti- Gout Therapy inflammatory Alprazolam Xanax Central Nervous Anxiolytics/Sedatives/Hypnotics System Amiodarone Cordarone Cardiovascular Antiarrhythmic Amitriptyline Elavil Central Nervous Antidepressant System Amlodipine Norvasc Cardiovascular Antihypertensive Amlodipine/Atorvastatin Caduet Cardiovascular Antihypertensive/Hypolipemic Amlodipine/Benazepril Lotrel Cardiovascular Antihypertensive Amoxicillin Amoxil Antimicrobial Antibiotic Amoxicillin/Clavulanate Augmentin Antimicrobial Antibiotic Amphetamine/Dextroamphetamine Adderall XR Central Nervous Stimulant System Aripiprazole Abilify Central Nervous Antipsychotic System Aspirin Bayer Analgesics/Anti- Non-Steroidal Anti- Aspirin inflammatory inflammatory/Anti-platelet Atenolol Tenormin Cardiovascular Antihypertensive Atorvastatin Lipitor Cardiovascular Hypolipemic Atropine Atropen Autonomic Antimuscarinic Azithromycin Zithromax Antimicrobial Antibiotic Beclomethasone Qvar Respiratory Topical Corticosteroid Benazepril Lotensin Cardiovascular Antihypertensive Bismuth Subsalicylate Pepto Gastrointestinal Acid/Peptic/Gastric Anti- Bismol -

The Effect of Pseudoephedrine on Blood Pressure in Patients With
The Effect Of Pseudoephedrine On Blood Pressure J Am Board Fam Pract: first published as 10.3122/jabfm.4.4.201 on 1 July 1991. Downloaded from In Patients With Controlled, Uncomplicated Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial John G. Bradley, M.D., Ken]. Kallai~ Ph.D., John N. Dorsch, M.D., andJoan Fox, R.N. Abslrtlet: Pseudoephedrine is frequendy used as a decongestant. Because of concern about 1be safety of pseudoephedrine in hypertensive patients, a clinical1rial was conducted to detennine whether blood pressure control was actuallya1fected by 1bis drug in a selected group of patients with hypertension. 1Wenty-nine patients wi1b controlled, uncomplicated hypertension, who received drug therapy and ranged in age from 25 to 50 years, were randomized to a treatment or a control group. Subjects took eI1ber 60 mg of pseudoephedrine or placebo capsules four times a day for 3 days. From 0800 hours until 2200 hours each day, the subjects obtained hourly blood pressure measurements using a porlable sphygmomanometer. An analysis of variance wi1b repeated measures was calculated to determine group differences for systolic and diastoUc readings. No statistically or clinically signiftcant differences were found. 1berapeu1ic: doses of pseudoephedrine did not adversely affect control of hypertension in 1bese selected patients. 0 Am Board Fam Pract 1991; 4:201-6.) Pseudoephedrine, phenylpropanolamine, and in hypertensive patients. While no adverse effect phenylephrine are oral sympathomimetic agents was found,IO this study was uncontrolled, a com commonly used as decongestants in prescription bination of drugs was used, and patient compli and over-the-counter preparations. Long-stand ance was unverified. -

Side Effects of Some Commonly Used Allergy Medications (Decongestants, Anti-Leukotriene Agents, Antihistamines, Steroids, and Zinc) and Their Safety in Pregnancy
ISSN: 2572-3308 Malone and Kennedy. Int J Aller Medications 2017, 3:024 DOI: 10.23937/2572-3308.1510024 Volume 3 | Issue 1 International Journal of Open Access Allergy Medications REVIEW ARTICLE Review: Side Effects of Some Commonly Used Allergy Medica- tions (Decongestants, Anti-Leukotriene Agents, Antihistamines, Steroids, and Zinc) and Their Safety in Pregnancy Michael Malone1* and Tara M Kennedy2 1Associate Professor and Medical Director, Department of Family and Community Medicine, Pennsylvania State University, USA 2Resident Physician, Department of Family and Community Medicine, Pennsylvania State University, USA *Corresponding author: Michael Malone, Associate Professor and Medical Director, Department of Family and Community Medicine, Pennsylvania State University, USA, E-mail: [email protected] Abstract Introduction Objective: The aim of this study was to evaluate the safety Allergic conditions are common, although most indi- and side-effects of common allergy medications. viduals with allergies do not seek medical care [1]. In the Methodology: A literature search was undertaken. We searched United States allergy medications are readily available the databases of Pubmed, MEDLINE, EMBASE databases, and over the counter. While most people will report relief of Cochrane Library from 1990 to October 2016, using key words: their symptoms with readily available allergy treatments, Allergy, Medications, Antihistamines, Decongestants, Montelu- approximately 7% of patients who take these medications kast, Side effects, Adverse Events, Adverse, Effects, Zinc, Ste- roids, Pregnancy, Reviews, RCT, and Case Report. will experience adverse effects (AEs), which can be severe [2]. This article will review side effects reported in the lit- Results: Antihistamines can cause undesired anti-cholinergic erature for some of the most common prescription and effects including mydraisais, sedation, dry eyes, dry mouth, con- stipation and urinary retention.