FDA Warns That Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm This Includes Heart and Mental Health Problems Or Death
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 2191
SECOND REGULAR SESSION HOUSE BILL NO. 2191 99TH GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE QUADE. 5582H.01I D. ADAM CRUMBLISS, Chief Clerk AN ACT To repeal section 579.060, RSMo, and to enact in lieu thereof one new section relating to controlled substances, with penalty provisions. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Section 579.060, RSMo, is repealed and one new section enacted in lieu 2 thereof, to be known as section 579.060, to read as follows: 579.060. 1. A person commits the offense of unlawful sale, distribution, or purchase of 2 over-the-counter methamphetamine precursor drugs if he or she knowingly: 3 (1) Sells, distributes, dispenses, or otherwise provides any number of packages of any 4 drug product containing detectable amounts of ephedrine, levomethamphetamine, 5 phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any of their salts, optical 6 isomers, or salts of optical isomers, in a total amount greater than nine grams to the same 7 individual within a thirty-day period, unless the amount is dispensed, sold, or distributed 8 pursuant to a valid prescription; or 9 (2) Purchases, receives, or otherwise acquires within a thirty-day period any number of 10 packages of any drug product containing any detectable amount of ephedrine, 11 levomethamphetamine, phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any 12 of their salts or optical isomers, or salts of optical isomers in a total amount greater than nine 13 grams, without regard to the number of transactions, unless the amount is purchased, received, 14 or acquired pursuant to a valid prescription; or 15 (3) Purchases, receives, or otherwise acquires within a twenty-four-hour period any 16 number of packages of any drug product containing any detectable amount of ephedrine, 17 levomethamphetamine, phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

The Stimulants and Hallucinogens Under Consideration: a Brief Overview of Their Chemistry and Pharmacology
Drug and Alcohol Dependence, 17 (1986) 107-118 107 Elsevier Scientific Publishers Ireland Ltd. THE STIMULANTS AND HALLUCINOGENS UNDER CONSIDERATION: A BRIEF OVERVIEW OF THEIR CHEMISTRY AND PHARMACOLOGY LOUIS S. HARRIS Dcparlmcnl of Pharmacology, Medical College of Virginia, Virginia Commonwealth Unwersity, Richmond, VA 23298 (U.S.A.) SUMMARY The substances under review are a heterogenous set of compounds from a pharmacological point of view, though many have a common phenylethyl- amine structure. Variations in structure lead to marked changes in potency and characteristic action. The introductory material presented here is meant to provide a set of chemical and pharmacological highlights of the 28 substances under con- sideration. The most commonly used names or INN names, Chemical Abstract (CA) names and numbers, and elemental formulae are provided in the accompanying figures. This provides both some basic information on the substances and a starting point for the more detailed information that follows in the individual papers by contributors to the symposium. Key words: Stimulants, their chemistry and pharmacology - Hallucinogens, their chemistry and pharmacology INTRODUCTION Cathine (Fig. 1) is one of the active principles of khat (Catha edulis). The structure has two asymmetric centers and exists as two geometric isomers, each of which has been resolved into its optical isomers. In the plant it exists as d-nor-pseudoephedrine. It is a typical sympathomimetic amine with a strong component of amphetamine-like activity. The racemic mixture is known generically in this country and others as phenylpropanolamine (dl- norephedrine). It is widely available as an over-the-counter (OTC) anti- appetite agent and nasal decongestant. -

I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St
2014;22(1):13-18 TCP Translational and Clinical Pharmacology http://dx.doi.org/10.12793/tcp.2014.22.1.13 Clinical Pharmacology Review for Primary Health Care Providers: I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul 137-701, Korea *Correspondence: S. Han; Tel: +82-2-2258-7326, Fax: +82-2-2258-7876, E-mail: [email protected] Received 31 May 2014 Primary health care providers play a critical role in maintaining public health, and the appropri- Accepted 31 May 2014 ate use of pharmaceutical products is one of the major parts of their practice. This series of articles, pISSN: 2289-0882 entitled ‘Clinical Pharmacology Review for Primary Health Care Providers,’ is intended to help pri- mary health care providers select more appropriate prescriptions for frequently used drugs based on up-to-date information. We expect that this effort will contribute to improvements in public health and diminish unnecessary drug use. Introduction tion on the H1 receptor.[8] THERAPEU Antihistamines include some of the most frequently prescribed drugs in the primary health care environment for the symp- Generations and Classes tomatic relief of allergic diseases, the common cold, urticaria, Many primary health care providers are well-informed about T and insomnia.[1-5] The importance of antihistamines has been the different ‘generations’ of antihistamines but not about the ICS TU emphasized as the prevalence of target diseases increases.[6,7] different ‘classes’ characterized according to chemical structure. However, the appropriate use, clinical effectiveness, and target [1] This discrepancy seems reasonable because ‘inter-generation’ T populations for prescription of antihistamines are still a matter differences are more prominent than ‘inter-class’ differences. -

The 2014 Prohibited List International Standard
The World Anti-Doping Code THE 2014 PROHIBITED LIST INTERNATIONAL STANDARD Version 2.0 (revised 2014 version) The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 September 2014 The revised 2014 Prohibited List 17 May 2014 THE 2014 PROHIBITED LIST WORLD ANTI-DOPING CODE Valid 1 September 2014 In accordance with Article 4.2.2 of the World Anti-Doping Code, all Prohibited Substances shall be considered as “Specified Substances” except Substances in classes S1, S2, S4.4, S4.5, S6.a, and Prohibited Methods M1, M2 and M3. SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) PROHIBITED SUBSTANCES S0. NON-APPROVED SUBSTANCES Any pharmacological substance which is not addressed by any of the subsequent sections of the List and with no current approval by any governmental regulatory health authority for human therapeutic use (e.g drugs under pre-clinical or clinical development or discontinued, designer drugs, substances approved only for veterinary use) is prohibited at all times. S1. ANABOLIC AGENTS Anabolic agents are prohibited. 1. Anabolic Androgenic Steroids (AAS) a. Exogenous* AAS, including: 1-androstenediol (5α-androst-1-ene-3β,17β-diol ); 1-androstenedione (5α- androst-1-ene-3,17-dione); bolandiol (estr-4-ene-3β,17β-diol ); bolasterone; boldenone; boldione (androsta-1,4-diene-3,17-dione); calusterone; clostebol; -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Pharmacology and Toxicology of Amphetamine and Related Designer Drugs
Pharmacology and Toxicology of Amphetamine and Related Designer Drugs U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alcohol Drug Abuse and Mental Health Administration Pharmacology and Toxicology of Amphetamine and Related Designer Drugs Editors: Khursheed Asghar, Ph.D. Division of Preclinical Research National Institute on Drug Abuse Errol De Souza, Ph.D. Addiction Research Center National Institute on Drug Abuse NIDA Research Monograph 94 1989 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, DC 20402 Pharmacology and Toxicology of Amphetamine and Related Designer Drugs ACKNOWLEDGMENT This monograph is based upon papers and discussion from a technical review on pharmacology and toxicology of amphetamine and related designer drugs that took place on August 2 through 4, 1988, in Bethesda, MD. The review meeting was sponsored by the Biomedical Branch, Division of Preclinical Research, and the Addiction Research Center, National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other matieral in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -
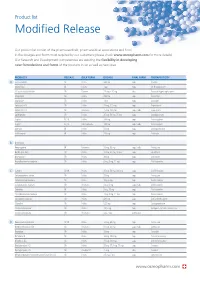
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Recommended Methods for the Identification and Analysis Of
Vienna International Centre, P.O. Box 500, 1400 Vienna, Austria Tel: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES Laboratory and Scientific Section United Nations Office on Drugs and Crime Vienna RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES UNITED NATIONS New York, 2006 Note Mention of company names and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. ST/NAR/34 UNITED NATIONS PUBLICATION Sales No. E.06.XI.1 ISBN 92-1-148208-9 Acknowledgements UNODC’s Laboratory and Scientific Section wishes to express its thanks to the experts who participated in the Consultative Meeting on “The Review of Methods for the Identification and Analysis of Amphetamine-type Stimulants (ATS) and Their Ring-substituted Analogues in Seized Material” for their contribution to the contents of this manual. Ms. Rosa Alis Rodríguez, Laboratorio de Drogas y Sanidad de Baleares, Palma de Mallorca, Spain Dr. Hans Bergkvist, SKL—National Laboratory of Forensic Science, Linköping, Sweden Ms. Warank Boonchuay, Division of Narcotics Analysis, Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand Dr. Rainer Dahlenburg, Bundeskriminalamt/KT34, Wiesbaden, Germany Mr. Adrian V. Kemmenoe, The Forensic Science Service, Birmingham Laboratory, Birmingham, United Kingdom Dr. Tohru Kishi, National Research Institute of Police Science, Chiba, Japan Dr. -

Whoexpertcommittee Ondrugdependence
Thisreportcontainsthecollectiveviewsof an international groupof expertsanddoesnotnecessarilyrepresentthedecisions Tile World 1lealth Organization is a specialized agcncyof the United Nations with orthestatedpolicyof theWorldHealthOrganization primary responsibility for international health'matters anti public health. Through this organization, which was created in 1948, the health professions of' some I65 countries exchange their knowledge and experience with the aim of making possible will permit them lo lead a socially and ccommlically productive lil_. WHOExpertCommittee By means of direct technical cooperation whh its Membcr Stales, and by stimt,- hhensiveding suchhealthcooperationservices, tamonghe preventionthem, WllOprornotesthcdevclopmcntorcomprc-anti control of diseascs, thc improvement o[ environmental conditions, tile development of health manpower, the coordination onDrugDependence anti development of biomedical and health services research, and thc planning and implementation of health programmes. These broad fiekls of endeavour encompass a wide variety of activities, such as developing systems of primary health cltre that reach the whole population of Mem- ber countries; promoting tile health of`mothers and children; combating malnutrition; controlling malaria and other communicable diseases including tuberculosis and leprosy; having achieved tile eradication or smallpox, promoting mass immunization against a numbcr of other preventable diseases; improving mental health; providing safe water supplies: anti training health -

Sudafed Decongestant Tablets 12S PIL SNAS 3359 SNAS 3557 14 Mm 29.07.2021 English Rate and Blood Pressure)
12 mm 12 mm spot = 12 x 2 centered in Distance between cutted the margin of 14 mm line/middel of the spot = 51.6 mm UK_Sudafed Decongestant Tablets 12s_PIL_SNAS 3359_SNAS 3557 14 mm 29.07.2021 English rate and blood pressure). 402059927_r2 N/A Now read this whole leaflet carefully before you use this medicine. Keep the leaflet: you ■ If you have an overactive thyroid Brochure/Leaet/Insert 145 x 250 mm might need it again. gland. 360165B BL-Val de Reuil_France ■ If you have glaucoma (increased CHILD 360165A Multi Packaging Solutions St Pierre 1 What the medicine is for pressure in the eye). 953 Oset Printing Sudafed Decongestant Tablets is a medicine used ■ If you have severe kidney problems. 8169503 PAPER to provide relief from cold, flu and allergy ■ If you are taking beta blockers (used to EMEA_2020_00006767_001 PC-0002778 symptoms such as blocked sinuses, stuffy nose treat high blood pressure). and catarrh. ■ If you are taking, or have taken in the The tablets contain pseudoephedrine last two weeks, drugs for depression ■ This medicine is used to help clear cold, flu hydrochloride, which is a decongestant that known as Monoamine Oxidase and allergy symptoms such as blocked relieves nasal and sinus congestion. Inhibitors (MAOIs) or Reversible Black PANTONE sinuses, stuffy nose and catarrh. This medicine is for use by adults and children Inhibitors of Monoamine Oxidase 3005 C ■ This medicine is for use by adults and (RIMAs). LITHO OFFSET LITHO OFFSET aged 12 years and over. children aged 12 years and over. 145 mm REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval. -

2008 Prohibited List
The World Anti-Doping Code THE 2008 PROHIBITED LIST INTERNATIONAL STANDARD The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2008 The Prohibited List 2008 September 22, 2007 THE 2008 PROHIBITED LIST WORLD ANTI-DOPING CODE Valid 1 January 2008 The use of any drug should be limited to medically justified indications SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) PROHIBITED SUBSTANCES S1. ANABOLIC AGENTS Anabolic agents are prohibited. 1. Anabolic Androgenic Steroids (AAS) a. Exogenous* AAS, including: 1-androstendiol (5α-androst-1-ene-3β,17β-diol ); 1-androstendione (5α- androst-1-ene-3,17-dione); bolandiol (19-norandrostenediol); bolasterone; boldenone; boldione (androsta-1,4-diene-3,17-dione); calusterone; clostebol; danazol (17α-ethynyl-17β-hydroxyandrost-4-eno[2,3-d]isoxazole); dehydrochlormethyltestosterone (4-chloro-17β-hydroxy-17α-methylandrosta- 1,4-dien-3-one); desoxymethyltestosterone (17α-methyl-5α-androst-2-en- 17β-ol); drostanolone; ethylestrenol (19-nor-17α-pregn-4-en-17-ol); fluoxymesterone; formebolone; furazabol (17β-hydroxy-17α-methyl-5α- androstano[2,3-c]-furazan); gestrinone; 4-hydroxytestosterone (4,17β- dihydroxyandrost-4-en-3-one); mestanolone; mesterolone; metenolone; methandienone (17β-hydroxy-17α-methylandrosta-1,4-dien-3-one); methandriol; methasterone (2α, 17α-dimethyl-5α-androstane-3-one-17β-ol);