I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacology
Pharmacology New for 2020-2021 Competitor orientation deleted from ILC. Event Summary Pharmacology provides HOSA members with the opportunity to gain knowledge and skills regarding the area of healthcare concerned with uses, effects, and modes of actions of drugs. This competitive event consists of a written test with a tie-breaker essay question. This event aims to inspire members to learn about how drugs work in the body, proper administration, and adaptations for different patients and conditions. Dress Code Competitors must be in official HOSA uniform or proper business attire. Bonus points will be awarded for proper dress. General Rules 1. Competitors in this event must be active members of HOSA-Future Health Professionals, in good standing. 2. Secondary and Postsecondary/Collegiate divisions are eligible to compete in this event. 3. Competitors must be familiar with and adhere to the “General Rules and Regulations of the HOSA Competitive Events Program (GRR)." 4. All competitors shall report to the site of the event at the time designated for each round of competition. At ILC, competitor’s photo ID must be presented prior to ALL competition rounds. Official References • Fulcher, Soto and Fulcher. Pharmacology: Principles and Applications. Elsevier, Latest edition. • Ford, Susan and Sally Roach. Roach’s Introductory Clinical Pharmacology. Wolters Kluwer, Latest edition. Written Test 5. Test Instructions: The written test will consist of 100 multiple choice items in a maximum of 90 minutes. 6. Time Remaining Announcements: There will be a verbal announcement when there are 60 minutes, 30 minutes, 15 minutes, 5 minutes, and 1 minute remaining to complete the test. -

Clinical Pharmacology 1: Phase 1 Studies and Early Drug Development
Clinical Pharmacology 1: Phase 1 Studies and Early Drug Development Gerlie Gieser, Ph.D. Office of Clinical Pharmacology, Div. IV Objectives • Outline the Phase 1 studies conducted to characterize the Clinical Pharmacology of a drug; describe important design elements of and the information gained from these studies. • List the Clinical Pharmacology characteristics of an Ideal Drug • Describe how the Clinical Pharmacology information from Phase 1 can help design Phase 2/3 trials • Discuss the timing of Clinical Pharmacology studies during drug development, and provide examples of how the information generated could impact the overall clinical development plan and product labeling. Phase 1 of Drug Development CLINICAL DEVELOPMENT RESEARCH PRE POST AND CLINICAL APPROVAL 1 DISCOVERY DEVELOPMENT 2 3 PHASE e e e s s s a a a h h h P P P Clinical Pharmacology Studies Initial IND (first in human) NDA/BLA SUBMISSION Phase 1 – studies designed mainly to investigate the safety/tolerability (if possible, identify MTD), pharmacokinetics and pharmacodynamics of an investigational drug in humans Clinical Pharmacology • Study of the Pharmacokinetics (PK) and Pharmacodynamics (PD) of the drug in humans – PK: what the body does to the drug (Absorption, Distribution, Metabolism, Excretion) – PD: what the drug does to the body • PK and PD profiles of the drug are influenced by physicochemical properties of the drug, product/formulation, administration route, patient’s intrinsic and extrinsic factors (e.g., organ dysfunction, diseases, concomitant medications, -

Guidance for Reviewers Pharmacology/Toxicology Review Format
Guidance for Reviewers Pharmacology/Toxicology Review Format U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Pharmacology/Toxicology May 2001 Guidance for Reviewers Pharmacology/Toxicology Review Format Additional copies are available from: Drug Information Branch (HFD-210) Center for Drug Evaluation and Research (CDER) 5600 Fishers Lane, Rockville, MD 20857, (Tel) 301-827-4570 Internet at http://www.fda.gov/der/guidance/index.htm U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Pharmacology/Toxicology May 2001 Guidance for Reviewers Table of Contents I. INTRODUCTION ....................................................................................................1 II. BACKGROUND.......................................................................................................1 III. DISCUSSION ...........................................................................................................1 A. IND REVIEW FORMAT ....................................................................................1 B. NDA REVIEW FORMAT...................................................................................1 IV. ATTACHMENTS .....................................................................................................1 A. IND REVIEW FORMAT ....................................................................................A-i B. NDA REVIEW FORMAT...................................................................................B-i -

Department of Pharmacology (GRAD) 1
Department of Pharmacology (GRAD) 1 faculty participate fully at all levels. The department has the highest DEPARTMENT OF level of NIH funding of all pharmacology departments and a great diversity of research areas is available to trainees. These areas PHARMACOLOGY (GRAD) include cell surface receptors, G proteins, protein kinases, and signal transduction mechanisms; neuropharmacology; nucleic acids, cancer, Contact Information and antimicrobial pharmacology; and experimental therapeutics. Cell and molecular approaches are particularly strong, but systems-level research Department of Pharmacology such as behavioral pharmacology and analysis of knock-in and knock-out Visit Program Website (http://www.med.unc.edu/pharm/) mice is also well-represented. Excellent physical facilities are available for all research areas. Henrik Dohlman, Chair Students completing the training program will have acquired basic The Department of Pharmacology offers a program of study that leads knowledge of pharmacology and related fields, in-depth knowledge in to the degree of doctor of philosophy in pharmacology. The curriculum is their dissertation research area, the ability to evaluate scientific literature, individualized in recognition of the diverse backgrounds and interests of mastery of a variety of laboratory procedures, skill in planning and students and the broad scope of the discipline of pharmacology. executing an important research project in pharmacology, and the ability The department offers a variety of research areas including to communicate results, analysis, and interpretation. These skills provide a sound basis for successful scientific careers in academia, government, 1. Receptors and signal transduction or industry. 2. Ion channels To apply to BBSP, students must use The Graduate School's online 3. -

Pharmacology 101
Pharmacology 101 Tyler Fischback, PharmD, BCPS, DPLA Clinical Pharmacy Manager Confluence Health Wenatchee, WA Objectives • Define Pharmacology, Pharmacokinetics and Pharmacodynamics • Understand how drug interactions work • Understand how some specific drugs behave in the body (opioids, benzodiazepines, amiodarone) • Apply pharmacology principles into practice Medication Errors and Adverse Drug Events • Error: An error of commission or omission at any step along the pathway that begins when a clinician prescribes a medication and ends when the patient actually received the medication. • ADE: Harm experienced by a patient as a result of exposure to a medication. ADE does not necessarily indicate an error or poor care. However, ~1/2 of ADEs are preventable. Anyone here ever seen a medication error or adverse drug event? Anyone here ever made a medication error? How many different prescription medications are available on the U.S. market? 1,000 So, it’s no surprise why we see so many problems………………… EXCEPT……. The real number is 10,000 Adverse Drug Events • ~1/3 of U.S. adults use 5 or more medications • Annually, ADE = 700,000 ER visits and 100,000 hospitalizations • So, is pharmacology important to your work? • Additionally, 5% of hospitalized patient experience an ADE during their stay • High risk: Anticoagulants, Opioids, Insulin, Cardiac, and Transitions of Care Adverse Drug Events, cont. • Elderly are more susceptible • Pediatrics patients more susceptible (weight-based dosing), especially liquids • Caregivers and patients admittedly -

FDA Warns That Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm This Includes Heart and Mental Health Problems Or Death
FDA warns that abuse and misuse of the nasal decongestant propylhexedrine causes serious harm This includes heart and mental health problems or death 3-25-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that the abuse and misuse of the over- the-counter (OTC) nasal decongestant propylhexedrine can lead to serious harm such as heart and mental health problems. Some of these complications, which include fast or abnormal heart rhythm, high blood pressure, and paranoia, can lead to hospitalization, disability, or death. Reports of individuals abusing and misusing propylhexedrine have increased in recent years. Propylhexedrine is safe and effective when used as directed. What is FDA doing? We are requesting that all manufacturers of OTC propylhexedrine nasal decongestant inhalers consider product design changes that support its safe use. For example, modifying the product to create a physical barrier that would make tampering with the device and abusing the propylhexedrine inside more difficult. In addition, decreasing the amount of medicine the device contains could also reduce the risk of serious side effects if abused or misused. We continue to evaluate this safety issue and will determine if additional FDA actions are needed. What is propylhexedrine and how can it help me? Propylhexedrine is a nasal decongestant that is available OTC in an inhaler. It is used short term to temporarily relieve nasal congestion due to colds, hay fever, or other upper respiratory allergies. It works by reducing swelling and inflammation of the mucous membrane lining of the nose. -
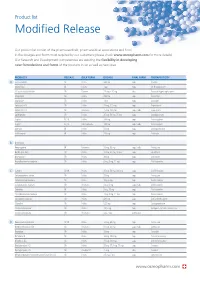
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Physiology, Biochemistry, and Pharmacology of Transporters for Organic Cations
International Journal of Molecular Sciences Editorial Physiology, Biochemistry, and Pharmacology of Transporters for Organic Cations Giuliano Ciarimboli Experimental Nephrology, Department of Internal Medicine D, University Hospital Münster, 48149 Münster, Germany; [email protected] This editorial summarizes the 13 scientific papers published in the Special Issue “Physiology, Biochemistry, and Pharmacology of Transporters for Organic Cations” of the International Journal of Molecular Sciences. In this Special Issue, the readers will find integrative information on transporters for organic cations. Besides reviews on physi- ology, pharmacology, and toxicology of these transporters [1–3], which offer a concise overview of the field, the readers will find original research work focusing on specific transporter aspects. Specifically, the review “Organic Cation Transporters in Human Physiology, Phar- macology, and Toxicology” by Samodelov et al. [1] summarizes well the general as- pects of physiology, pharmacology, and toxicology of transporter for organic cations. The other review “Organic Cation Transporters in the Lung—Current and Emerging (Patho)Physiological and Pharmacological Concepts” by Ali Selo et al. [2] focuses on these aspects of transporters for organic cations in the lung, an important but often ne- glected field. In the paper “Systems Biology Analysis Reveals Eight SLC22 Transporter Subgroups, Including OATs, OCTs, and OCTNs”, by performing a system biology analysis of SLC22 transporters, Engelhart et al. [4] suggest the existence of a transporter–metabolite network. They propose that, in this network, mono-, oligo-, and multi-specific SLC22 transporters Citation: Ciarimboli, G. Physiology, interact to regulate concentrations and fluxes of many metabolites and signaling molecules. Biochemistry, and Pharmacology of In particular, the organic cation transporters (OCT) subgroup seems to be associated with Transporters for Organic Cations. -

Sudafed Decongestant Tablets 12S PIL SNAS 3359 SNAS 3557 14 Mm 29.07.2021 English Rate and Blood Pressure)
12 mm 12 mm spot = 12 x 2 centered in Distance between cutted the margin of 14 mm line/middel of the spot = 51.6 mm UK_Sudafed Decongestant Tablets 12s_PIL_SNAS 3359_SNAS 3557 14 mm 29.07.2021 English rate and blood pressure). 402059927_r2 N/A Now read this whole leaflet carefully before you use this medicine. Keep the leaflet: you ■ If you have an overactive thyroid Brochure/Leaet/Insert 145 x 250 mm might need it again. gland. 360165B BL-Val de Reuil_France ■ If you have glaucoma (increased CHILD 360165A Multi Packaging Solutions St Pierre 1 What the medicine is for pressure in the eye). 953 Oset Printing Sudafed Decongestant Tablets is a medicine used ■ If you have severe kidney problems. 8169503 PAPER to provide relief from cold, flu and allergy ■ If you are taking beta blockers (used to EMEA_2020_00006767_001 PC-0002778 symptoms such as blocked sinuses, stuffy nose treat high blood pressure). and catarrh. ■ If you are taking, or have taken in the The tablets contain pseudoephedrine last two weeks, drugs for depression ■ This medicine is used to help clear cold, flu hydrochloride, which is a decongestant that known as Monoamine Oxidase and allergy symptoms such as blocked relieves nasal and sinus congestion. Inhibitors (MAOIs) or Reversible Black PANTONE sinuses, stuffy nose and catarrh. This medicine is for use by adults and children Inhibitors of Monoamine Oxidase 3005 C ■ This medicine is for use by adults and (RIMAs). LITHO OFFSET LITHO OFFSET aged 12 years and over. children aged 12 years and over. 145 mm REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval. -

110499 Calcium-Antagonist Drugs
DRUG THERAPY Review Article Drug Therapy smooth muscle (arteriolar and venous), nonvascular smooth muscle (bronchial, gastrointestinal, genitouri- nary, and uterine), and noncontractile tissues (pan- A LASTAIR J.J. WOOD, M.D., Editor creas, pituitary, adrenal glands, salivary glands, gastric mucosa, white cells, platelets, and lacrimal tissue). Blockade of L-type channels in vascular tissues results CALCIUM-ANTAGONIST DRUGS in the relaxation of vascular smooth muscle and in cardiac tisssue results in a negative inotropic effect. DARRELL R. ABERNETHY, M.D., PH.D., The ability of these drugs to decrease smooth-muscle AND JANICE B. SCHWARTZ, M.D. and myocardial contractility results in both clinically desirable antihypertensive and antianginal effects and undesirable myocardial depression. RUGS classified as calcium antagonists or Other calcium channels with electrophysiologic calcium-channel blockers were introduced properties have also been identified. These channels, into clinical medicine in the 1960s and are to which the calcium antagonists do not bind, in- D clude the N-type channels in neuronal tissue, P-type now among the most frequently prescribed drugs for the treatment of cardiovascular diseases.1 Although channels in Purkinje tissues, and T-type (transient potential) channels in cardiac nodal structures and the currently available calcium antagonists are chem- 4,5 ically diverse, they share the common property of vascular smooth muscle. blocking the transmembrane flow of calcium ions Regulation of the L-type channels may differ in through voltage-gated L-type (slowly inactivating) different types of cells. In cardiac myocytes, these channels.2 These drugs have proved effective in pa- channels are activated by catecholamines and other stimuli that activate adenylyl cyclase or cyclic aden- tients with hypertension, angina pectoris, and cardi- 6-8 ac arrhythmias and may be beneficial in patients with osine monophosphate–dependent protein kinase. -

Histamine and Antihistamines Sites of Action Conditions Which Cause Release Aron H
Learning Objectives I Histamine Pharmacological effects Histamine and Antihistamines Sites of action Conditions which cause release Aron H. Lichtman, Ph.D. Diagnostic uses Associate Professor II Antihistamines acting at the H1 and H2 receptor Pharmacology and Toxicology Pharmacological effects Mechanisms of action Therapeutic uses Side effects and drug interactions Be familiar with the existence of the H3 receptor III Be able to describe the main mechanism of action of cromolyn sodium and its clinical uses Histamine Pharmacology First autacoid to be discovered. (Greek: autos=self; Histamine Formation akos=cure) Synthesized in 1907 Synthesized in mammalian tissues by Demonstrated to be a natural constituent of decarboxylation of the amino acid l-histidine mammalian tissues (1927) Involved in inflammatory and anaphylactic reactions. Local application causes swelling redness, and edema, mimicking a mild inflammatory reaction. Large systemic doses leads to profound vascular changes similar to those seen after shock or anaphylactic origin Histamine Stored in complex with: Heparin Chondroitin Sulfate Eosinophilic Chemotactic Factor Neutrophilic Chemotactic Factor Proteases 1 Conditions That Release Histamine 1. Tissue injury: Any physical or chemical agent that injures tissue, skin or mucosa are particularly sensitive to injury and will cause the immediate release of histamine from mast cells. 2. Allergic reactions: exposure of an antigen to a previously sensitized (exposed) subject can immediately trigger allergic reactions. If sensitized by IgE antibodies attached to their surface membranes will degranulate when exposed to the appropriate antigen and release histamine, ATP and other mediators. 3. Drugs and other foreign compounds: morphine, dextran, antimalarial drugs, dyes, antibiotic bases, alkaloids, amides, quaternary ammonium compounds, enzymes (phospholipase C). -

Calcium Channel Blockers in Cardiovascular Pharmacotherapy
Cardiovascular Pharmacology Core Review Journal of Cardiovascular Pharmacology and Therapeutics 2014, Vol. 19(6) 501-515 Calcium Channel Blockers in ª The Author(s) 2014 Reprints and permission: Cardiovascular Pharmacotherapy sagepub.com/journalsPermissions.nav DOI: 10.1177/1074248414530508 cpt.sagepub.com Theophile Godfraind1 Abstract This paper summarizes the pharmacological properties of calcium channel blockers (CCBs), their established therapeutic uses for cardiovascular disorders and the current improvement of their clinical effects through drug combinations. Their identification resulted from study of small molecules including coronary dilators, which were named calcium antagonists. Further experiments showed that they reduced contraction of arteries by inhibiting calcium entry and by interacting with binding sites identified on voltage-dependent calcium channels. This led to the denomination calcium channel blockers. In short-term studies, by decreasing total peripheral resistance, CCBs lower arterial pressure. By unloading the heart and increasing coronary blood flow, CCBs improve myocardial oxygenation. In long-term treatment, the decrease in blood pressure is more pronounced in hypertensive than in normotensive patients. A controversy on the safety of CCBs ended after a large antihypertensive trial (ALLHAT) sponsored by the National Heart, Lung, and Blood Institute. There are two main types of CCBs: dihydopyridine and non-dihydropyridine; the first type is vascular selective. Dihydropyrines are indicated for hypertension, chronic, stable and vasospastic angina. Non-dihydropyridines have the same indications plus antiarrythmic effects in atrial fibrillation or flutter and paroxysmal supraventricular tachycardia. In addition, CCBs reduced newly formed coronary lesions in atherosclerosis. In order to reach recommended blood pressure goals, there is a recent therapeutic move by combination of CCBs with other antihypertensive agents particularly with inhibitors acting at the level of the renin-angiotensin system.