Lergy, Bronchodilator, and Antiasthmatic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacotherapeutic Considerations for Individuals with Down Syndrome Erik Hefti
Harrisburg University of Science and Technology Digital Commons at Harrisburg University Harrisburg University Faculty Works 12-8-2016 Pharmacotherapeutic Considerations for Individuals with Down Syndrome Erik Hefti Follow this and additional works at: http://digitalcommons.harrisburgu.edu/faculty-works Part of the Congenital, Hereditary, and Neonatal Diseases and Abnormalities Commons, and the Medicinal and Pharmaceutical Chemistry Commons R EVIEW O F T HERAPEUTICS Pharmacotherapeutic Considerations for Individuals with Down Syndrome Erik Hefti,* and Javier G. Blanco* Department of Pharmaceutical Sciences, The School of Pharmacy and Pharmaceutical Sciences, The State University of New York at Buffalo, Buffalo, New York Down syndrome (DS; trisomy 21) is the most common survivable disorder due to aneuploidy. Individ- uals with DS may experience multiple comorbid health problems including congenital heart defects, endocrine abnormalities, skin and dental problems, seizure disorders, leukemia, dementia, and obesity. These associated conditions may necessitate pharmacotherapeutic management with various drugs. The complex pathobiology of DS may alter drug disposition and drug response in some individuals. For example, reports have documented increased rates of adverse drug reactions in patients with DS treated for leukemia and dementia. Intellectual disability resulting from DS may impact adherence to medication regimens. In this review, we highlight literature focused on pharmacotherapy for individu- als with DS. We discuss reports of altered drug disposition or response in patients with DS and explore social factors that may impact medication adherence in the DS setting. Enhanced monitoring during drug therapy in individuals with DS is justified based on reports of altered drug disposition, drug response, and other characteristics present in this population. -

I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St
2014;22(1):13-18 TCP Translational and Clinical Pharmacology http://dx.doi.org/10.12793/tcp.2014.22.1.13 Clinical Pharmacology Review for Primary Health Care Providers: I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul 137-701, Korea *Correspondence: S. Han; Tel: +82-2-2258-7326, Fax: +82-2-2258-7876, E-mail: [email protected] Received 31 May 2014 Primary health care providers play a critical role in maintaining public health, and the appropri- Accepted 31 May 2014 ate use of pharmaceutical products is one of the major parts of their practice. This series of articles, pISSN: 2289-0882 entitled ‘Clinical Pharmacology Review for Primary Health Care Providers,’ is intended to help pri- mary health care providers select more appropriate prescriptions for frequently used drugs based on up-to-date information. We expect that this effort will contribute to improvements in public health and diminish unnecessary drug use. Introduction tion on the H1 receptor.[8] THERAPEU Antihistamines include some of the most frequently prescribed drugs in the primary health care environment for the symp- Generations and Classes tomatic relief of allergic diseases, the common cold, urticaria, Many primary health care providers are well-informed about T and insomnia.[1-5] The importance of antihistamines has been the different ‘generations’ of antihistamines but not about the ICS TU emphasized as the prevalence of target diseases increases.[6,7] different ‘classes’ characterized according to chemical structure. However, the appropriate use, clinical effectiveness, and target [1] This discrepancy seems reasonable because ‘inter-generation’ T populations for prescription of antihistamines are still a matter differences are more prominent than ‘inter-class’ differences. -

DEMAND REDUCTION a Glossary of Terms
UNITED NATIONS PUBLICATION Sales No. E.00.XI.9 ISBN: 92-1-148129-5 ACKNOWLEDGEMENTS This document was prepared by the: United Nations International Drug Control Programme (UNDCP), Vienna, Austria, in consultation with the Commonwealth of Health and Aged Care, Australia, and the informal international reference group. ii Contents Page Foreword . xi Demand reduction: A glossary of terms . 1 Abstinence . 1 Abuse . 1 Abuse liability . 2 Action research . 2 Addiction, addict . 2 Administration (method of) . 3 Adverse drug reaction . 4 Advice services . 4 Advocacy . 4 Agonist . 4 AIDS . 5 Al-Anon . 5 Alcohol . 5 Alcoholics Anonymous (AA) . 6 Alternatives to drug use . 6 Amfetamine . 6 Amotivational syndrome . 6 Amphetamine . 6 Amyl nitrate . 8 Analgesic . 8 iii Page Antagonist . 8 Anti-anxiety drug . 8 Antidepressant . 8 Backloading . 9 Bad trip . 9 Barbiturate . 9 Benzodiazepine . 10 Blood-borne virus . 10 Brief intervention . 11 Buprenorphine . 11 Caffeine . 12 Cannabis . 12 Chasing . 13 Cocaine . 13 Coca leaves . 14 Coca paste . 14 Cold turkey . 14 Community empowerment . 15 Co-morbidity . 15 Comprehensive Multidisciplinary Outline of Future Activities in Drug Abuse Control (CMO) . 15 Controlled substance . 15 Counselling and psychotherapy . 16 Court diversion . 16 Crash . 16 Cross-dependence . 17 Cross-tolerance . 17 Custody diversion . 17 Dance drug . 18 Decriminalization or depenalization . 18 Demand . 18 iv Page Demand reduction . 19 Dependence, dependence syndrome . 19 Dependence liability . 20 Depressant . 20 Designer drug . 20 Detoxification . 20 Diacetylmorphine/Diamorphine . 21 Diuretic . 21 Drug . 21 Drug abuse . 22 Drug abuse-related harm . 22 Drug abuse-related problem . 22 Drug policy . 23 Drug seeking . 23 Drug substitution . 23 Drug testing . 24 Drug use . -

FDA Warns That Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm This Includes Heart and Mental Health Problems Or Death
FDA warns that abuse and misuse of the nasal decongestant propylhexedrine causes serious harm This includes heart and mental health problems or death 3-25-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that the abuse and misuse of the over- the-counter (OTC) nasal decongestant propylhexedrine can lead to serious harm such as heart and mental health problems. Some of these complications, which include fast or abnormal heart rhythm, high blood pressure, and paranoia, can lead to hospitalization, disability, or death. Reports of individuals abusing and misusing propylhexedrine have increased in recent years. Propylhexedrine is safe and effective when used as directed. What is FDA doing? We are requesting that all manufacturers of OTC propylhexedrine nasal decongestant inhalers consider product design changes that support its safe use. For example, modifying the product to create a physical barrier that would make tampering with the device and abusing the propylhexedrine inside more difficult. In addition, decreasing the amount of medicine the device contains could also reduce the risk of serious side effects if abused or misused. We continue to evaluate this safety issue and will determine if additional FDA actions are needed. What is propylhexedrine and how can it help me? Propylhexedrine is a nasal decongestant that is available OTC in an inhaler. It is used short term to temporarily relieve nasal congestion due to colds, hay fever, or other upper respiratory allergies. It works by reducing swelling and inflammation of the mucous membrane lining of the nose. -
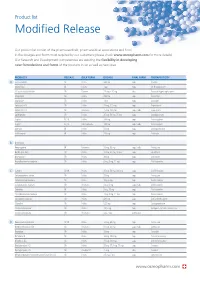
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Effectiveness of Bronchodilator and Corticosteroid Treatment in Patients with Chronic Obstructive Pulmonary Disease (Copd)
Journal of Pharmaceutical Science and Application Volume 2, Issue 1, Page 17-22, June 2020 E-ISSN : 2301-7708 EFFECTIVENESS OF BRONCHODILATOR AND CORTICOSTEROID TREATMENT IN PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) Putu Rika Veryanti1*, Ainun Wulandari1 1Department of Pharmacy, Institut Sains dan Teknologi Nasional, Jakarta, Indonesia Corresponding author email: [email protected] ABSTRACT Background: Chronic Obstructive Pulmonary Disease (COPD) is a chronic airway disease which is characterized by progressive airway obstruction. Bronchodilators and corticosteroids are the first choices of therapy in COPD patients. The goal therapy of COPD patients is to prevent respiratory failure, which can impact on death. But nowadays, the mortality rate due to COPD continues to increase. WHO predicts mortality from COPD in the year 2030 will be ranked third in the world. This high mortality can be caused by the ineffectiveness of therapy given. Objective: The aim of this study is to find out the effectiveness of bronchodilator and corticosteroid treatments in COPD patients. Methods: An observational study conducted retrospectively in the 2018 period at Fatmawati Central General Hospital. The effectiveness of therapy was assessed from the patient's clinical condition, blood gas values (PaO2 & PaCO2) and the average length of stay (AvLOS). Results: COPD was mostly suffered by males (83,33%), and the highest age for COPD was in the range of 45 years and above (90%). Bronchodilator that commonly prescribed were albuterol (30.08%), ipratropium bromide (12.2%), fenoterol hydrobromide (10.57%), terbutaline sulfate (8.13%), theophylline (1.63%) and aminophylline (5.69%), while the corticosteroids were budesonide (17.07%), methylprednisolone (9.76%) and dexamethasone (4.88%). -

Sudafed Decongestant Tablets 12S PIL SNAS 3359 SNAS 3557 14 Mm 29.07.2021 English Rate and Blood Pressure)
12 mm 12 mm spot = 12 x 2 centered in Distance between cutted the margin of 14 mm line/middel of the spot = 51.6 mm UK_Sudafed Decongestant Tablets 12s_PIL_SNAS 3359_SNAS 3557 14 mm 29.07.2021 English rate and blood pressure). 402059927_r2 N/A Now read this whole leaflet carefully before you use this medicine. Keep the leaflet: you ■ If you have an overactive thyroid Brochure/Leaet/Insert 145 x 250 mm might need it again. gland. 360165B BL-Val de Reuil_France ■ If you have glaucoma (increased CHILD 360165A Multi Packaging Solutions St Pierre 1 What the medicine is for pressure in the eye). 953 Oset Printing Sudafed Decongestant Tablets is a medicine used ■ If you have severe kidney problems. 8169503 PAPER to provide relief from cold, flu and allergy ■ If you are taking beta blockers (used to EMEA_2020_00006767_001 PC-0002778 symptoms such as blocked sinuses, stuffy nose treat high blood pressure). and catarrh. ■ If you are taking, or have taken in the The tablets contain pseudoephedrine last two weeks, drugs for depression ■ This medicine is used to help clear cold, flu hydrochloride, which is a decongestant that known as Monoamine Oxidase and allergy symptoms such as blocked relieves nasal and sinus congestion. Inhibitors (MAOIs) or Reversible Black PANTONE sinuses, stuffy nose and catarrh. This medicine is for use by adults and children Inhibitors of Monoamine Oxidase 3005 C ■ This medicine is for use by adults and (RIMAs). LITHO OFFSET LITHO OFFSET aged 12 years and over. children aged 12 years and over. 145 mm REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval. -

Dr. Duke's Phytochemical and Ethnobotanical Databases Biological Activities Found in Ammi Visnaga
Dr. Duke's Phytochemical and Ethnobotanical Databases Biological Activities found in Ammi visnaga Activity Chemical Count 11B-HSD-Inhibitor 2 5-Alpha-Reductase-Inhibitor 3 5-HT-Inhibitor 1 5-Lipoxygenase-Inhibitor 2 ACE-Inhibitor 3 Acetylcholinergic 1 Acidulant 1 Aldehyde-Oxidase-Inhibitor 2 Aldose-Reductase-Inhibitor 11 Allelochemic 5 Allelopathic 3 Allergenic 8 Alpha-Reductase-Inhibitor 1 Analgesic 5 Anemiagenic 1 Anesthetic 3 Anthelmintic 1 Antiacetylcholinesterase 2 Antiacne 3 AntiADD 1 Antiaflatoxin 6 Antiaggregant 5 Antiaging 1 Antiallergenic 1 Antiallergic 5 Antialopecic 2 Antialzheimeran 1 Activity Chemical Count Antianaphylactic 2 Antiandrogenic 3 Antianginal 1 Antiangiogenic 1 Antiapoplectic 1 Antiappetant 1 Antiarteriosclerotic 1 Antiarthritic 1 Antiasthmatic 4 Antiatherogenic 1 Antiatherosclerotic 3 Antibacterial 15 Antibrucellosic 1 Anticancer 6 Anticancer (Kidney) 1 Anticancer (Prostate) 1 Anticapillary-Fragility 1 Anticarcinomic (Breast) 1 Anticariogenic 2 Anticataract 2 Anticlastogen 1 Anticolitic 1 Anticomplementary 1 Anticonvulsant 3 AntiCrohn's 1 AntiCVI 1 Anticystitic 1 2 Activity Chemical Count Antidementia 1 Antidepressant 2 Antidermatitic 3 Antidiabetic 4 Antidiarrheic 1 Antidiuretic 1 Antidote (Camphor) 1 Antidote (Morphine) 1 Antidysenteric 1 Antiedemic 3 Antielastase 2 Antiemetic 1 Antiencephalitic 1 Antierythemic 1 Antiescherichic 2 Antiesherichic 1 Antiestrogenic 2 Antifeedant 7 Antifertility 1 Antifibrinolytic 1 Antifibrosarcomic 1 Antifibrositic 1 Antiflu 1 Antigastric 2 Antigingivitic 3 Antiglaucomic 1 Antigonadotrophic -

Histamine and Antihistamines Sites of Action Conditions Which Cause Release Aron H
Learning Objectives I Histamine Pharmacological effects Histamine and Antihistamines Sites of action Conditions which cause release Aron H. Lichtman, Ph.D. Diagnostic uses Associate Professor II Antihistamines acting at the H1 and H2 receptor Pharmacology and Toxicology Pharmacological effects Mechanisms of action Therapeutic uses Side effects and drug interactions Be familiar with the existence of the H3 receptor III Be able to describe the main mechanism of action of cromolyn sodium and its clinical uses Histamine Pharmacology First autacoid to be discovered. (Greek: autos=self; Histamine Formation akos=cure) Synthesized in 1907 Synthesized in mammalian tissues by Demonstrated to be a natural constituent of decarboxylation of the amino acid l-histidine mammalian tissues (1927) Involved in inflammatory and anaphylactic reactions. Local application causes swelling redness, and edema, mimicking a mild inflammatory reaction. Large systemic doses leads to profound vascular changes similar to those seen after shock or anaphylactic origin Histamine Stored in complex with: Heparin Chondroitin Sulfate Eosinophilic Chemotactic Factor Neutrophilic Chemotactic Factor Proteases 1 Conditions That Release Histamine 1. Tissue injury: Any physical or chemical agent that injures tissue, skin or mucosa are particularly sensitive to injury and will cause the immediate release of histamine from mast cells. 2. Allergic reactions: exposure of an antigen to a previously sensitized (exposed) subject can immediately trigger allergic reactions. If sensitized by IgE antibodies attached to their surface membranes will degranulate when exposed to the appropriate antigen and release histamine, ATP and other mediators. 3. Drugs and other foreign compounds: morphine, dextran, antimalarial drugs, dyes, antibiotic bases, alkaloids, amides, quaternary ammonium compounds, enzymes (phospholipase C). -

262 Part 341—Cold, Cough, Al- Lergy, Bronchodilator, And
Pt. 341 21 CFR Ch. I (4–1–18 Edition) section 502 of the Act relating to mis- treating concurrent symptoms (in either branding and the prohibition in section a single-ingredient or combination drug 301(d) of the Act against the introduc- product). tion or delivery for introduction into 341.72 Labeling of antihistamine drug prod- interstate commerce of unapproved ucts. 341.74 Labeling of antitussive drug prod- new drugs in violation of section 505(a) ucts. of the Act. 341.76 Labeling of bronchodilator drug prod- (c) Warnings. The labeling of the ucts. product contains the following warn- 341.78 Labeling of expectorant drug prod- ings under the heading ‘‘Warnings’’: ucts. (1) ‘‘The recommended dose of this 341.80 Labeling of nasal decongestant drug product contains about as much caf- products. feine as a cup of coffee. Limit the use 341.85 Labeling of permitted combinations of caffeine-containing medications, of active ingredients. foods, or beverages while taking this 341.90 Professional labeling. product because too much caffeine may AUTHORITY: 21 U.S.C. 321, 351, 352, 353, 355, cause nervousness, irritability, sleep- 360, 371. lessness, and, occasionally, rapid heart EDITORIAL NOTE: Nomenclature changes to beat.’’ part 341 appear at 69 FR 13717, Mar. 24, 2004. (2) ‘‘For occasional use only. Not in- tended for use as a substitute for sleep. Subpart A—General Provisions If fatigue or drowsiness persists or con- tinues to recur, consult a’’ (select one § 341.1 Scope. of the following: ‘‘physician’’ or ‘‘doc- tor’’). (a) An over-the-counter cold, cough, (3) ‘‘Do not give to children under 12 allergy, bronchodilator, or anti- years of age.’’ asthmatic drug product in a form suit- (d) Directions. -

Mucolytic Agents & Decongestants
Mucolytic Agents & Decongestants Mucolytic agents (Guaifenesin Mucinex® Humibid LA®) are drugs that thin mucus and secretions, so they can drain out of the sinuses more easily. They may be helpful for people suffering from a thick post-nasal drip. Often, they are found in combination preparations with decongestants and/or antihistamines. Most are well tolerated and have few side effects such as headache, rash, or nausea. Topical Nasal Decongestants Topical nasal decongestants (Afrin® or Neosynephrine®) in the form of drops or sprays that are placed in the nose can be very effective in shrinking nasal swelling in minutes. However, these sprays should be used no longer than 2 or 3 consecutive days, for prolonged usage may result in "rebound" swelling of the nose. Rebound swelling (known as "rhinitis medicamentosa") can be extremely difficult to treat because the nose may no longer respond normally to the decongestant spray. We advise that use the brands that contain oxymetazoline in preference to those that have phenylephrine as their active ingredient. The safe use of these medications sometimes can be extended by using the medicine only at bedtime, in alternating nostrils every other night. For example, on the first night only the right nostril receives medicine and on the second night the medicine is placed in your left nostril. By doing this some patients can double the safe use of oxymetazoline. Systemic Decongestants If more prolonged decongestant therapy is required, systemic decongestants may be used. The most well know brand is Sudafed®. It comes in varying strengths and is purchased over the counter. Psuedoephedrine can befound as part of a combination medication with some antihistamines such as Allegra® or Claritin®. -

Desoxyn (Methamphetamine Hydrochloride Tablets, USP)
® Desoxyn (methamphetamine hydrochloride tablets, USP) Rx only METHAMPHETAMINE HAS A HIGH POTENTIAL FOR ABUSE. IT SHOULD THUS BE TRIED ONLY IN WEIGHT REDUCTION PROGRAMS FOR PATIENTS IN WHOM ALTERNATIVE THERAPY HAS BEEN INEFFECTIVE. ADMINISTRATION OF METHAMPHETAMINE FOR PROLONGED PERIODS OF TIME IN OBESITY MAY LEAD TO DRUG DEPENDENCE AND MUST BE AVOIDED. PARTICULAR ATTENTION SHOULD BE PAID TO THE POSSIBILITY OF SUBJECTS OBTAINING METHAMPHETAMINE FOR NON-THERAPEUTIC USE OR DISTRIBUTION TO OTHERS, AND THE DRUG SHOULD BE PRESCRIBED OR DISPENSED SPARINGLY. MISUSE OF METHAMPHETAMINE MAY CAUSE SUDDEN DEATH AND SERIOUS CARDIOVASCULAR ADVERSE EVENTS. DESCRIPTION DESOXYN® (methamphetamine hydrochloride tablets, USP), chemically known as (S)-N,α-dimethylbenzeneethanamine hydrochloride, is a member of the amphetamine group of sympathomimetic amines. It has the following structural formula: DESOXYN tablets contain 5 mg of methamphetamine hydrochloride for oral administration. Inactive Ingredients: Corn starch, lactose, sodium paraminobenzoate, stearic acid and talc. CLINICAL PHARMACOLOGY Methamphetamine is a sympathomimetic amine with CNS stimulant activity. Peripheral actions include elevation of systolic and diastolic blood pressures and weak bronchodilator and respiratory stimulant action. Drugs of this class used in obesity are commonly known as “anorectics” or “anorexigenics”. It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions, or metabolic effects, may be involved, for example. Reference ID: 3734642 Adult obese subjects instructed in dietary management and treated with “anorectic” drugs, lose more weight on the average than those treated with placebo and diet, as determined in relatively short-term clinical trials. The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week.