The Effect of Pseudoephedrine on Blood Pressure in Patients With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 2191
SECOND REGULAR SESSION HOUSE BILL NO. 2191 99TH GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE QUADE. 5582H.01I D. ADAM CRUMBLISS, Chief Clerk AN ACT To repeal section 579.060, RSMo, and to enact in lieu thereof one new section relating to controlled substances, with penalty provisions. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Section 579.060, RSMo, is repealed and one new section enacted in lieu 2 thereof, to be known as section 579.060, to read as follows: 579.060. 1. A person commits the offense of unlawful sale, distribution, or purchase of 2 over-the-counter methamphetamine precursor drugs if he or she knowingly: 3 (1) Sells, distributes, dispenses, or otherwise provides any number of packages of any 4 drug product containing detectable amounts of ephedrine, levomethamphetamine, 5 phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any of their salts, optical 6 isomers, or salts of optical isomers, in a total amount greater than nine grams to the same 7 individual within a thirty-day period, unless the amount is dispensed, sold, or distributed 8 pursuant to a valid prescription; or 9 (2) Purchases, receives, or otherwise acquires within a thirty-day period any number of 10 packages of any drug product containing any detectable amount of ephedrine, 11 levomethamphetamine, phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any 12 of their salts or optical isomers, or salts of optical isomers in a total amount greater than nine 13 grams, without regard to the number of transactions, unless the amount is purchased, received, 14 or acquired pursuant to a valid prescription; or 15 (3) Purchases, receives, or otherwise acquires within a twenty-four-hour period any 16 number of packages of any drug product containing any detectable amount of ephedrine, 17 levomethamphetamine, phenylpropanolamine, propylhexedrine, or pseudoephedrine, or any EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

Hypertension – Adult – Clinical Practice Guideline
Hypertension – Adult – Clinical Practice Guideline Table of Contents EXECUTIVE SUMMARY ........................................................................................................... 3 SCOPE ...................................................................................................................................... 4 METHODOLOGY ...................................................................................................................... 5 INTRODUCTION ....................................................................................................................... 5 RECOMMENDATIONS .............................................................................................................. 5 Establish the Diagnosis ........................................................................................... 5 Patient Evaluation ................................................................................................... 7 Treatment Goals ..................................................................................................... 7 Lifestyle Modifications ............................................................................................. 8 Table 4 – Lifestyle Modifications ........................................................................ 9 Medication Treatment............................................................................................ 11 Figure 1 - Initiation and Titration of Antihypertensive Medication ..................... 13 Table 7 - Antihypertensive -

The Stimulants and Hallucinogens Under Consideration: a Brief Overview of Their Chemistry and Pharmacology
Drug and Alcohol Dependence, 17 (1986) 107-118 107 Elsevier Scientific Publishers Ireland Ltd. THE STIMULANTS AND HALLUCINOGENS UNDER CONSIDERATION: A BRIEF OVERVIEW OF THEIR CHEMISTRY AND PHARMACOLOGY LOUIS S. HARRIS Dcparlmcnl of Pharmacology, Medical College of Virginia, Virginia Commonwealth Unwersity, Richmond, VA 23298 (U.S.A.) SUMMARY The substances under review are a heterogenous set of compounds from a pharmacological point of view, though many have a common phenylethyl- amine structure. Variations in structure lead to marked changes in potency and characteristic action. The introductory material presented here is meant to provide a set of chemical and pharmacological highlights of the 28 substances under con- sideration. The most commonly used names or INN names, Chemical Abstract (CA) names and numbers, and elemental formulae are provided in the accompanying figures. This provides both some basic information on the substances and a starting point for the more detailed information that follows in the individual papers by contributors to the symposium. Key words: Stimulants, their chemistry and pharmacology - Hallucinogens, their chemistry and pharmacology INTRODUCTION Cathine (Fig. 1) is one of the active principles of khat (Catha edulis). The structure has two asymmetric centers and exists as two geometric isomers, each of which has been resolved into its optical isomers. In the plant it exists as d-nor-pseudoephedrine. It is a typical sympathomimetic amine with a strong component of amphetamine-like activity. The racemic mixture is known generically in this country and others as phenylpropanolamine (dl- norephedrine). It is widely available as an over-the-counter (OTC) anti- appetite agent and nasal decongestant. -

I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St
2014;22(1):13-18 TCP Translational and Clinical Pharmacology http://dx.doi.org/10.12793/tcp.2014.22.1.13 Clinical Pharmacology Review for Primary Health Care Providers: I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul 137-701, Korea *Correspondence: S. Han; Tel: +82-2-2258-7326, Fax: +82-2-2258-7876, E-mail: [email protected] Received 31 May 2014 Primary health care providers play a critical role in maintaining public health, and the appropri- Accepted 31 May 2014 ate use of pharmaceutical products is one of the major parts of their practice. This series of articles, pISSN: 2289-0882 entitled ‘Clinical Pharmacology Review for Primary Health Care Providers,’ is intended to help pri- mary health care providers select more appropriate prescriptions for frequently used drugs based on up-to-date information. We expect that this effort will contribute to improvements in public health and diminish unnecessary drug use. Introduction tion on the H1 receptor.[8] THERAPEU Antihistamines include some of the most frequently prescribed drugs in the primary health care environment for the symp- Generations and Classes tomatic relief of allergic diseases, the common cold, urticaria, Many primary health care providers are well-informed about T and insomnia.[1-5] The importance of antihistamines has been the different ‘generations’ of antihistamines but not about the ICS TU emphasized as the prevalence of target diseases increases.[6,7] different ‘classes’ characterized according to chemical structure. However, the appropriate use, clinical effectiveness, and target [1] This discrepancy seems reasonable because ‘inter-generation’ T populations for prescription of antihistamines are still a matter differences are more prominent than ‘inter-class’ differences. -

Pseudoephedrine Smurfing Fuels Surge in Large-Scale
ARCHIVED LIMITED OFFICIAL USE-LAW ENFORCEMENT SENSITIVE//FOR OFFICIAL USE ONLY U.S. Department of Justice National Drug Intelligence Center June 2009 Situation Report Product No. 2009-S0787-007 Pseudoephedrine Smurfing Fuels Surge in Large-Scale Methamphetamine Production in California Preface This Situation Report is in response to a request from the Office of National Drug Control Policy for information regarding pseudoephedrine smurfing in California. The National Drug Intelligence Center collected and analyzed data and reporting from 2007 through May 2009 related to metham- phetamine production and pseudoephedrine smurfing. This report draws upon data from the National Seizure System (NSS) as well as information obtained through interviews with federal, state, and local law enforcement officers. Executive Summary Pseudoephedrine smurfinga has become increasingly organized and widespread in California, par- ticularly since 2007, fueling an increase in the number of large-scale methamphetamine laboratories in the state.1 Among the increased number of large-scale laboratories are those operated by Mexican criminal groups that have relocated to California from Mexico since 2007. Mexican criminal groups and some independent operators are increasingly acquiring bulk quantities of pseudoephedrine through smurfing. Despite strong efforts by law enforcement to curtail smurfing, there is no indication that this practice will decline in the near term. In fact, pseudoephedrine acquired through smurfing in California in 2009 was sent in bulk to methamphetamine producers in Mexico, an indication that some criminal groups in Mexico still find it easier to acquire pseudoephedrine through smurfing in California than from other sources. Discussion Pseudoephedrine smurfing increased significantly in California in 2008 and early 2009. -

Unit 10 Shock,Resuscitation Part A
Vanderbilt University Medical Center Emergency General Surgery Service Surgical Residency Rotation and Curriculum UNIT 10SHOCK, RESUSCITATION, AND SURGICAL CRITICAL CARE PART A: SHOCK AND RESUSCITATION UNIT OBJECTIVES: 1. Demonstrate an understanding of the pathophysiology of shock and its categories. 2. Demonstrate an understanding of the mechanisms and pathophysiology of cardiopulmonary arrest. 3. Demonstrate the ability to manage the treatment of shock and cardiopulmonary arrest. COMPETENCY-BASED KNOWLEDGE OBJECTIVES: 1. Define the categories of shock based upon type, and explain the etiology and pathophysiology of each type of shock: a. Cardiogenic b. Hypovolemic c. Distributive (septic, anaphylactic, neurogenic, and adrenal insufficiency mediated) d. Obstructive (cardiac tamponade, tension pneumothorax, pulmonary embolus) 2. Summarize the clinical presentation and hemodynamic parameters associated with each type of shock. 3. Propose an algorithm for diagnosing and initiating treatment for each shock type. 4. Discuss the pathophysiology, including the mechanism of arrest, for each of the following situations: a. Acute myocardial infarction f. Substance abuse b. Acute dysrhythmia g. Hypothermia c. Congestive heart failure h. Acute stroke d. Pulmonary embolus i. Hemorrhagic shock e. Tension pneumothorax 5. Explain the indications for and the pharmacokinetics of each of the following drugs: a. Lidocaine g. Quinidine b. Bretylium h. Isoproterenol c. Digoxin i. Amiodarone d. Propanolol j. Dopamine e. Verapamil k. Dobutamine f. Pronestyl l. Adenosine (Adenocard®) 6. Summarize the indications and the appropriate techniques for cardioversion and defibrillation. Vanderbilt University Medical Center Emergency General Surgery Service Surgical Residency Rotation and Curriculum 7. Outline the signs and symptoms of acute airway obstruction and define the appropriate intervention in adult and pediatric patients. -

Methamphetamine Manufactured from Pseudoephedrine; Both As Double
2019 Methamphetamine Manufactured from Pseudoephedrine; Both as Double- Edged Swords Fanak Fahimi* *Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Received: 2019-12-20, Revised: 2019-12-21, Accept: 2019-12-22, Published: 2019-12-31 A R T I C L E I N F O Article type: Editorial J Pharm Care 2019; 7(4):80-81. Please cite this paper as: Fahimi F. Methamphetamine Manufactured from Pseudoephedrine; Both as Double-Edged Swords. J Pharm Care 2019; 7(4): 80-81. Methamphetamine, or meth, is a potent stimulant that meth. The regulation was effective from September 2006 is prescribed for psychiatric conditions such as attention (8). So, by law the following were required: deficit hyperactivity disorder (ADHD) and weight issues. All pseudoephedrine containing OTC medicines should Contrariwise, this agent is more used recreationally for be sold from behind a sales counter and limits of 3.6 energy boosting and feelings of strength it creates. grams per day or 9 grams per 30 days. The medicine is Pseudoephedrine (PSE) is the key ingredient used to only sold to patient who presents a formal identification produce methamphetamine. Pseudoephedrine has one card and provide personal information which will be kept less hydroxyl group compared to another commonly used in the pharmacy for 2 years. sympathomimetic in cold and allergy products which Meth lab numbers dropped by more than 65% in 2007 results in a more lipid soluble molecule and thus more following this law. Sadly, meth lab events remained high or central nervous system (CNS) availability (1). -

FDA Warns That Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm This Includes Heart and Mental Health Problems Or Death
FDA warns that abuse and misuse of the nasal decongestant propylhexedrine causes serious harm This includes heart and mental health problems or death 3-25-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that the abuse and misuse of the over- the-counter (OTC) nasal decongestant propylhexedrine can lead to serious harm such as heart and mental health problems. Some of these complications, which include fast or abnormal heart rhythm, high blood pressure, and paranoia, can lead to hospitalization, disability, or death. Reports of individuals abusing and misusing propylhexedrine have increased in recent years. Propylhexedrine is safe and effective when used as directed. What is FDA doing? We are requesting that all manufacturers of OTC propylhexedrine nasal decongestant inhalers consider product design changes that support its safe use. For example, modifying the product to create a physical barrier that would make tampering with the device and abusing the propylhexedrine inside more difficult. In addition, decreasing the amount of medicine the device contains could also reduce the risk of serious side effects if abused or misused. We continue to evaluate this safety issue and will determine if additional FDA actions are needed. What is propylhexedrine and how can it help me? Propylhexedrine is a nasal decongestant that is available OTC in an inhaler. It is used short term to temporarily relieve nasal congestion due to colds, hay fever, or other upper respiratory allergies. It works by reducing swelling and inflammation of the mucous membrane lining of the nose. -
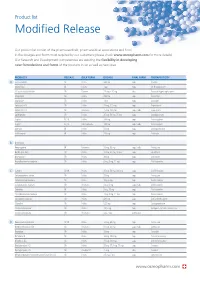
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Recommended Methods for the Identification and Analysis Of
Vienna International Centre, P.O. Box 500, 1400 Vienna, Austria Tel: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES Laboratory and Scientific Section United Nations Office on Drugs and Crime Vienna RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES UNITED NATIONS New York, 2006 Note Mention of company names and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. ST/NAR/34 UNITED NATIONS PUBLICATION Sales No. E.06.XI.1 ISBN 92-1-148208-9 Acknowledgements UNODC’s Laboratory and Scientific Section wishes to express its thanks to the experts who participated in the Consultative Meeting on “The Review of Methods for the Identification and Analysis of Amphetamine-type Stimulants (ATS) and Their Ring-substituted Analogues in Seized Material” for their contribution to the contents of this manual. Ms. Rosa Alis Rodríguez, Laboratorio de Drogas y Sanidad de Baleares, Palma de Mallorca, Spain Dr. Hans Bergkvist, SKL—National Laboratory of Forensic Science, Linköping, Sweden Ms. Warank Boonchuay, Division of Narcotics Analysis, Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand Dr. Rainer Dahlenburg, Bundeskriminalamt/KT34, Wiesbaden, Germany Mr. Adrian V. Kemmenoe, The Forensic Science Service, Birmingham Laboratory, Birmingham, United Kingdom Dr. Tohru Kishi, National Research Institute of Police Science, Chiba, Japan Dr. -

Sudafed Decongestant Tablets 12S PIL SNAS 3359 SNAS 3557 14 Mm 29.07.2021 English Rate and Blood Pressure)
12 mm 12 mm spot = 12 x 2 centered in Distance between cutted the margin of 14 mm line/middel of the spot = 51.6 mm UK_Sudafed Decongestant Tablets 12s_PIL_SNAS 3359_SNAS 3557 14 mm 29.07.2021 English rate and blood pressure). 402059927_r2 N/A Now read this whole leaflet carefully before you use this medicine. Keep the leaflet: you ■ If you have an overactive thyroid Brochure/Leaet/Insert 145 x 250 mm might need it again. gland. 360165B BL-Val de Reuil_France ■ If you have glaucoma (increased CHILD 360165A Multi Packaging Solutions St Pierre 1 What the medicine is for pressure in the eye). 953 Oset Printing Sudafed Decongestant Tablets is a medicine used ■ If you have severe kidney problems. 8169503 PAPER to provide relief from cold, flu and allergy ■ If you are taking beta blockers (used to EMEA_2020_00006767_001 PC-0002778 symptoms such as blocked sinuses, stuffy nose treat high blood pressure). and catarrh. ■ If you are taking, or have taken in the The tablets contain pseudoephedrine last two weeks, drugs for depression ■ This medicine is used to help clear cold, flu hydrochloride, which is a decongestant that known as Monoamine Oxidase and allergy symptoms such as blocked relieves nasal and sinus congestion. Inhibitors (MAOIs) or Reversible Black PANTONE sinuses, stuffy nose and catarrh. This medicine is for use by adults and children Inhibitors of Monoamine Oxidase 3005 C ■ This medicine is for use by adults and (RIMAs). LITHO OFFSET LITHO OFFSET aged 12 years and over. children aged 12 years and over. 145 mm REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval. -

1This Action Arises Under the Patent Laws of the United States. See 35 U.S.C
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA MCNEIL-PPC, INC., : Plaintiff, : CIVIL ACTION : v. : : L. PERRIGO COMPANY, : and PERRIGO COMPANY, : No. 01-1100 Defendants. : OPINION AND ORDER SCHILLER, J. June , 2002 This is a patent infringement action. Plaintiff McNeil-PPC, Inc. (“McNeil”) alleges Defendants L. Perrigo Company and Perrigo Company (collectively “Perrigo”) infringe four McNeil patents covering a popular version of the Imodium® Advanced antidiarrheal. In a Memorandum and Order issued April 3, 2002, I construed certain disputed claim terms pursuant to Markman v. Westview Instruments, Inc. , 517 U.S. 370 (1996). Beginning April 22, 2002, this matter was tried without a jury, and I enter the following Findings of Fact and Conclusions of Law as required by Rule 52(a) of the Federal Rules of Civil Procedure. 1 FINDINGS OF FACT I. BACKGROUND This action pits a manufacturer of national brand pharmaceuticals against its competitor, a generic drug manufacturer. Four patents owned by Plaintiff McNeil are at issue in this case: United States Patents 5,248,505 (“the ’505 patent”)(PTX1) and 5,612,054 (“the ’054 patent”)(PTX2) are 1This action arises under the patent laws of the United States. See 35 U.S.C. § 271(e)(2) and 21 U.S.C. § 355(j). Jurisdiction is based on 28 U.S.C. §§ 1331 and 1338(a). Venue is proper in this Court pursuant to 28 U.S.C. §§ 1391(c) and 1400(b). referred to as “the Garwin patents”; 2 United States Patents 5,679,376 (“the ’376 patent”)(PTX3) and 5,716,641 (“the ’641 patent”)(PTX4) are referred to as “the Stevens patents.” A.