Generalized Hyperhidrosis Secondary to Presumed Eccrine Gland Dysfunction with Possible Apocrine Metaplasia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skates and Rays Diversity, Exploration and Conservation – Case-Study of the Thornback Ray, Raja Clavata
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Doutoramento em Ciências do Mar 2010 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Tese orientada por Professor Auxiliar com Agregação Leonel Serrano Gordo e Investigadora Auxiliar Ivone Figueiredo Doutoramento em Ciências do Mar 2010 The research reported in this thesis was carried out at the Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB), Unidade de Recursos Marinhos e Sustentabilidade. This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through a PhD grant (SFRH/BD/23777/2005) and the research project EU Data Collection/DCR (PNAB). Skates and rays diversity, exploration and conservation | Table of Contents Table of Contents List of Figures ............................................................................................................................. i List of Tables ............................................................................................................................. v List of Abbreviations ............................................................................................................. viii Agradecimentos ........................................................................................................................ -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Embryology and Development of Salivary Gland 1
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 10, 2020 Embryology and development of salivary gland 1. Dr. Sangeetha Priya.P,Dr.N.Anitha, Dr.E.Rajesh, Dr.K.M.K.Masthan Professor, Department of Oral Pathology and Microbiology Sree Balaji Dental College and Hospital Bharath Institute of Higher Education and Research ABSTRACT: Saliva is the mixed glandular secretion which constantly bathes the teeth and the oral mucosa. It is constituted by the secretions of the three paired major salivary glands; the parotid, submandibular and sublingual . Salivary glands are complex in nature. They could be either tubulo acinar, merocrine or exocrine glands secreting mainly saliva. Salivary gland is one of the main soft tissue structures in the maxillofacial area. This review article illustrates the processes that lead to the development,embryology of the salivary glands and how this relates to the adult anatomy. KEY WORDS: salivary gland embryology, salivary gland development, salivary gland anatomy INTRODUCTION: A gland consists of specialized type of cells, wherein they produce products which are used elsewhere in the body. Salivary glands are complex, tubulo acinar, exocrine or merocrine glands secreting mainly saliva. Saliva is the product of the major and minor salivary gland dispersed throughout the oral cavity. It is a complex mixture of organic, inorganic components and water, carrying out several functions. There are three pairs of major salivary glands namely parotid, sub mandibular and sublingual glands in addition to numerous minor salivary glands in the oral cavity1. Together these glands produce saliva, which contains digestive enzymes, antibodies, growth factors, and coating substances essential for eating, speaking, tasting, and oral hygiene2,3. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -

Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr
Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr. Heather Yule ([email protected]) October 21, 2014 Slides for study: 75 (Salivary Gland), 355 (Pancreas Tail), 48 (Atrophic Mammary Gland), 49 (Active Mammary Gland) and 50 (Resting Mammary Gland) Electron micrographs for : study EM: Serous acinus in parotid gland EM: Mucous acinus in mixed salivary gland EM: Pancreatic acinar cell Main Objective: Understand key histological features of glandular epithelium and relate structure to function. Specific Objectives: 1. Describe key histological differences between endocrine and exocrine glands including their development. 2. Compare three modes of secretion in glands; holocrine, apocrine and merocrine. 3. Explain the functional significance of polarization of glandular epithelial cells. 4. Define the terms parenchyma, stroma, mucous acinus, serous acinus and serous a demilune and be able to them identify in glandular tissue. 5. Distinguish exocrine and endocrine pancreas. 6. Compare the histology of resting, lactating and postmenopausal mammary glands. Keywords: endocrine gland, exocrine gland, holocrine, apocrine, merocrine, polarity, parenchyma, stroma, acinus, myoepithelial cell, mucous gland, serous gland, mixed or seromucous gland, serous demilune, exocrine pancreas, endocrine pancreas (pancreatic islets), resting mammary gland, lactating mammary gland, postmenopausal mammary gland “This copy is made solely for your personal use for research, private study, education, parody, satire, criticism, or review -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -
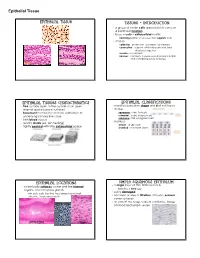
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

A Focus on Breast Cancer
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Pharmacol Manuscript Author Ther. Author Manuscript Author manuscript; available in PMC 2017 May 01. Published in final edited form as: Pharmacol Ther. 2016 May ; 161: 79–96. doi:10.1016/j.pharmthera.2016.03.003. Emerging therapeutic targets in metastatic progression: a focus on breast cancer Zhuo Li and Yibin Kang* Department of Molecular Biology, Princeton University, Princeton, NJ, 08544, United States Abstract Metastasis is the underlying cause of death for the majority of breast cancer patients. Despite significant advances in recent years in basic research and clinical development, therapies that specifically target metastatic breast cancer remain inadequate, and represents the single greatest obstacle to reducing mortality of late-stage breast cancer. Recent efforts have leveraged genomic analysis of breast cancer and molecular dissection of tumor-stromal cross-talk to uncover a number of promising candidates for targeted treatment of metastatic breast cancer. Rational combinations of therapeutic agents targeting tumor-intrinsic properties and microenvironmental components provide a promising strategy to develop precision treatments with higher specificity and less toxicity. In this review, we discuss the emerging therapeutic targets in breast cancer metastasis, from tumor-intrinsic pathways to those that involve the host tissue components, including the immune system. Keywords Breast cancer; Metastasis; Targeted therapy; Tumor microenvironment; Immunotherapy 1. Introduction The overall 5-year survival rate for breast cancer currently stands at 90% — a dramatic improvement over the 63% survival rate in the early 1960s. When stratified by stage, the 5- year survival rates have increased to 99% for localized disease and 85% for regional advanced disease, a trend that can be attributed to early diagnoses and better treatment regimens. -

Squamous Epithelium in the Human Thyroid Gland
J Clin Pathol: first published as 10.1136/jcp.19.4.384 on 1 July 1966. Downloaded from J. clin. Path. (1966), 19, 384. Squamous epithelium in the human thyroid gland J. N. HARCOURT-WEBSTER1 From the Department ofPathology, University ofEdinburgh SYNOPSIS Four cases are reported in each of which squamous epithelium was an incidental finding in surgically excised thyroid gland tissue. The occasional thyroid cyst lined throughout by squamous cells probably represents a persistent ultimo-branchial body, but the evidence indicates that the usual source of such cells in this gland is metaplasia of the follicular epithelium. An explanation is offered for the infrequency of this transformation in the thyroid, despite the frequent occurrence of the changes which predispose to epithelial metaplasia at other sites. There is no evidence to suggest that squamous cells arising in this gland by either of these means have any sinister significance. Squamous epithelium was first described in a human iodine uptake studies were normal but serological anti- thyroid gland by Nicholson (1922); he attributed body tests were positive (ADT: +ve on second day; this finding to metaplasia of the follicular epithelium TCH: 1/250,000; CFT: 1/250). The provisional diagnosis was Hashimoto's disease; a biopsy was taken from the induced by severe chronic inflammatory and fibrotic right lobe with a wedge resection of the isthmus. changes in the gland. Epithelial metaplasia occurs in Histology Sections of the rock-hard, pale fawn tissue response to altered function or at least as the result show abundant, interwoven, thick bands of hyaline col- of altered environment (Boyd, 1961). -

Mixed Type Adrenal Cyst: a Case Report
Eastern Journal of Medicine 20 (2015) 163-166 Case Report Mixed type adrenal cyst: A case report Gulay Buluta,*, Mehmet Deniz Bulutb, Deniz Yilmaza, Sebahattin Celikc, Nursen Toprakb aDepartment of Pathology, Yuzuncu Yil University Faculty of Medicine, Van, Turkey bDepartment of Radiology, Van Research and Training Hospital, Van, Turkey cDepartment of General Surgery, Van Research and Training Hospital, Van, Turkey Abstract. Adrenal cysts are rare lesions that usually progress asymptomatically. They are often determined post- mortem. Our case was a 50-year old-male who had presented to our clinic with upper right quadrant pain. The computed tomography demonstrated a cystic mass in the adrenal gland. No abnormalities were determined in the laboratory tests. According to the histopathological and immunohistochemical examination, the cyst was evaluated as a mixed-type adrenal cyst, the wall of which was covered with endothelium, mesothelium and cubic epithelium. We believed that the case was worth presenting, since it was the first diagnosed mixed-type adrenal cyst in the literature. Key words: Mixed-type adrenal cyst, endothelial cyst, epithelial cyst, mesothelial cyst pain. There was no history of hypertension or 1. Introduction trauma in the past. Per abdominal examination Adrenal cysts are rarely encountered clinical showed a soft abdomen; no mass was felt. entities. Their incidence has been reported from Haemogram was normal. A complete endocrine 0.06%-0.18% in autopsy series (1). In 1966, workup including the estimation of urinary Foster put forth a classification based on the metanephrines, vanillylmandelic acid and free histopathology of 220 adrenal cysts into parasitic cortisol did not show any hormonal cysts (7%), epithelial cysts (9%), pseudocysts hypersecretion.