Johnson Noise and Shot Noise: the Determination of the Boltzmann Constant, Absolute Zero Temperature and the Charge of the Electron
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Jitter and Wander Measurements and Standards Second Edition Contents
Understanding Jitter and Wander Measurements and Standards Second Edition Contents Page Preface 1. Introduction to Jitter and Wander 1-1 2. Jitter Standards and Applications 2-1 3. Jitter Testing in the Optical Transport Network 3-1 (OTN) 4. Jitter Tolerance Measurements 4-1 5. Jitter Transfer Measurement 5-1 6. Accurate Measurement of Jitter Generation 6-1 7. How Tester Intrinsics and Transients 7-1 affect your Jitter Measurement 8. What 0.172 Doesn’t Tell You 8-1 9. Measuring 100 mUIp-p Jitter Generation with an 0.172 Tester? 9-1 10. Faster Jitter Testing with Simultaneous Filters 10-1 11. Verifying Jitter Generator Performance 11-1 12. An Overview of Wander Measurements 12-1 Acronyms Welcome to the second edition of Agilent Technologies Understanding Jitter and Wander Measurements and Standards booklet, the distillation of over 20 years’ experience and know-how in the field of telecommunications jitter testing. The Telecommunications Networks Test Division of Agilent Technologies (formerly Hewlett-Packard) in Scotland introduced the first jitter measurement instrument in 1982 for PDH rates up to E3 and DS3, followed by one of the first 140 Mb/s jitter testers in 1984. SONET/SDH jitter test capability followed in the 1990s, and recently Agilent introduced one of the first 10 Gb/s Optical Channel jitter test sets for measurements on the new ITU-T G.709 frame structure. Over the years, Agilent has been a significant participant in the development of jitter industry standards, with many contributions to ITU-T O.172/O.173, the standards for jitter test equipment, and Telcordia GR-253/ ITU-T G.783, the standards for operational SONET/SDH network equipment. -

Retrospective Analysis of Phonatory Outcomes After CO2 Laser Thyroarytenoid 00111 Myoneurectomy in Patients with Adductor Spasmodic Dysphonia
Global Journal of Otolaryngology ISSN 2474-7556 Research Article Glob J Otolaryngol Volume 22 Issue 4- June 2020 Copyright © All rights are reserved by Rohan Bidaye DOI: 10.19080/GJO.2020.22.556091 Retrospective Analysis of Phonatory Outcomes after CO2 Laser Thyroarytenoid Myoneurectomy in Patients with Adductor Spasmodic Dysphonia Rohan Bidaye1*, Sachin Gandhi2*, Aishwarya M3 and Vrushali Desai4 1Senior Clinical Fellow in Laryngology, Deenanath Mangeshkar Hospital, India 2Head of Department of ENT, Deenanath Mangeshkar Hospital, India 3Fellow in Laryngology, Deenanath Mangeshkar Hospital, India 4Chief Consultant, Speech Language Pathologist, Deenanath Mangeshkar Hospital, India Submission: May 16, 2020; Published: June 08, 2020 *Corresponding author: Rohan Bidaye and Sachin Gandhi, Senior Clinical Fellow in Laryngology and Head of Department of ENT, Deenanath Mangeshkar Hospital, India Abstract Introduction: Adductor spasmodic dysphonia (ADSD) is a focal laryngeal dystonia characterized by spasms of laryngeal muscles during speech. Botulinum toxin injection in the Thyroarytenoid muscle remains the gold-standard treatment for ADSD. However, as Botulinum toxin CO2 laser Thyroarytenoid myoneurectomy (TAM) has been reported as an effective technique for treatment of ADSD. It provides sustained improvementinjections need in tothe be voice repeated over a periodically,longer duration. the voice quality fluctuates over a longer period. A Microlaryngoscopic Transoral approach to Methods: Trans oral Microlaryngoscopic CO2 laser TAM was performed in 14 patients (5 females and 9 males), aged between 19 and 64 years who were diagnosed with ADSD. Data was collected from over 3 years starting from Jan 2014 – Dec 2016. GRBAS scale along with Multi- dimensional voice programme (MDVP) analysis of the voice and Video laryngo-stroboscopic (VLS) samples at the end of 3 and 12 months of surgery would be compared with the pre-operative readings. -

Perturbation and Harmonics to Noise Ratio As a Function of Gender in the Aged Voice
Perturbation and Harmonics to Noise Ratio as a Function of Gender in the Aged Voice THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Arts in the Graduate School of The Ohio State University By Meredith Margaret Rouse Hunt Graduate Program in Speech and Hearing Science The Ohio State University 2012 Master's Examination Committee: Michael Trudeau, Advisor Michelle Bourgeois Copyrighted by Meredith Margaret Rouse Hunt 2012 Abstract The purpose of this investigation was to explore possible differences as a function of gender in perturbation (jitter and shimmer) and harmonics to noise ratio (HNR) among aged male and female speakers. Thirty normal aged adults (15 males; 15 females; over age 60) prolonged the vowel /a/ at a comfortable loudness level. Measures of jitter (%), shimmer (%), and HNR were used to compare vocal function between aged gender groups. No significant differences were found between genders on any of the measures. Findings are discussed relative to other published studies on similar measures and support data that aged voices exhibit increased variability. Future suggestions for research are discussed. ii Dedication This manuscript is dedicated to my husband, Ryan, for his unfailing patience, support, and humor during the completion of my thesis and in all aspects of my life. iii Acknowledgments I would like to acknowledge Michael Trudeau, Ph. D., CCC-SLP, my academic and thesis advisor, for his gentle and persistent guidance. His dedication to teaching and patience with students has allowed me to become adept at critical evaluations of research and treatment methodology. More importantly, his love of voice science and care for his clients has shaped my future professional career as speech-language pathologist. -

22Nd International Congress on Acoustics ICA 2016
Page intentionaly left blank 22nd International Congress on Acoustics ICA 2016 PROCEEDINGS Editors: Federico Miyara Ernesto Accolti Vivian Pasch Nilda Vechiatti X Congreso Iberoamericano de Acústica XIV Congreso Argentino de Acústica XXVI Encontro da Sociedade Brasileira de Acústica 22nd International Congress on Acoustics ICA 2016 : Proceedings / Federico Miyara ... [et al.] ; compilado por Federico Miyara ; Ernesto Accolti. - 1a ed . - Gonnet : Asociación de Acústicos Argentinos, 2016. Libro digital, PDF Archivo Digital: descarga y online ISBN 978-987-24713-6-1 1. Acústica. 2. Acústica Arquitectónica. 3. Electroacústica. I. Miyara, Federico II. Miyara, Federico, comp. III. Accolti, Ernesto, comp. CDD 690.22 ISBN 978-987-24713-6-1 © Asociación de Acústicos Argentinos Hecho el depósito que marca la ley 11.723 Disclaimer: The material, information, results, opinions, and/or views in this publication, as well as the claim for authorship and originality, are the sole responsibility of the respective author(s) of each paper, not the International Commission for Acoustics, the Federación Iberoamaricana de Acústica, the Asociación de Acústicos Argentinos or any of their employees, members, authorities, or editors. Except for the cases in which it is expressly stated, the papers have not been subject to peer review. The editors have attempted to accomplish a uniform presentation for all papers and the authors have been given the opportunity to correct detected formatting non-compliances Hecho en Argentina Made in Argentina Asociación de Acústicos Argentinos, AdAA Camino Centenario y 5006, Gonnet, Buenos Aires, Argentina http://www.adaa.org.ar Proceedings of the 22th International Congress on Acoustics ICA 2016 5-9 September 2016 Catholic University of Argentina, Buenos Aires, Argentina ICA 2016 has been organised by the Ibero-american Federation of Acoustics (FIA) and the Argentinian Acousticians Association (AdAA) on behalf of the International Commission for Acoustics. -

Johnson Noise Thermometry Measurement of the Boltzmann Constant with a 200 Ω Sense Resistor Alessio Pollarolo, Taehee Jeong, Samuel P
1512 IEEE TRANSACTIONS ON INSTRUMENTATION AND MEASUREMENT, VOL. 62, NO. 6, JUNE 2013 Johnson Noise Thermometry Measurement of the Boltzmann Constant With a 200 Ω Sense Resistor Alessio Pollarolo, Taehee Jeong, Samuel P. Benz, Senior Member, IEEE, and Horst Rogalla, Member, IEEE Abstract—In 2010, the National Institute of Standards and Technology measured the Boltzmann constant k with an electronic technique that measured the Johnson noise of a 100 Ω resistor at the triple point of water and used a voltage waveform synthesized with a quantized voltage noise source (QVNS) as a reference. In this paper, we present measurements of k using a 200 Ω sense re- sistor and an appropriately modified QVNS circuit and waveform. Preliminary results show agreement with the previous value within the statistical uncertainty. An analysis is presented, where the largest source of uncertainty is identified, which is the frequency dependence in the constant term a0 of the two-parameter fit. Index Terms—Boltzmann equation, Josephson junction, mea- surement units, noise measurement, standards, temperature. Fig. 1. Schematic diagram of the Johnson-noise two-channel cross-correlator. I. INTRODUCTION HE Johnson–Nyquist equation (1) defines the thermal measurement electronics are calibrated by using a pseudonoise T noise power (Johnson noise) V 2 of a resistor in a voltage waveform synthesized with the quantized voltage noise bandwidth Δf through its resistance R and its thermodynamic source (QVNS) that acts as a spectral-density reference [8], [9]. temperature T [1], [2]: Fig. 1 shows the experimental schematic. The two chan- nels of the cross-correlator simultaneously amplify, filter, and 2 VR =4kTRΔf. -

PROCEEDINGS of the ICA CONGRESS (Onl the ICA PROCEEDINGS OF
ine) - ISSN 2415-1599 ISSN ine) - PROCEEDINGS OF THE ICA CONGRESS (onl THE ICA PROCEEDINGS OF Page intentionaly left blank 22nd International Congress on Acoustics ICA 2016 PROCEEDINGS Editors: Federico Miyara Ernesto Accolti Vivian Pasch Nilda Vechiatti X Congreso Iberoamericano de Acústica XIV Congreso Argentino de Acústica XXVI Encontro da Sociedade Brasileira de Acústica 22nd International Congress on Acoustics ICA 2016 : Proceedings / Federico Miyara ... [et al.] ; compilado por Federico Miyara ; Ernesto Accolti. - 1a ed . - Gonnet : Asociación de Acústicos Argentinos, 2016. Libro digital, PDF Archivo Digital: descarga y online ISBN 978-987-24713-6-1 1. Acústica. 2. Acústica Arquitectónica. 3. Electroacústica. I. Miyara, Federico II. Miyara, Federico, comp. III. Accolti, Ernesto, comp. CDD 690.22 ISSN 2415-1599 ISBN 978-987-24713-6-1 © Asociación de Acústicos Argentinos Hecho el depósito que marca la ley 11.723 Disclaimer: The material, information, results, opinions, and/or views in this publication, as well as the claim for authorship and originality, are the sole responsibility of the respective author(s) of each paper, not the International Commission for Acoustics, the Federación Iberoamaricana de Acústica, the Asociación de Acústicos Argentinos or any of their employees, members, authorities, or editors. Except for the cases in which it is expressly stated, the papers have not been subject to peer review. The editors have attempted to accomplish a uniform presentation for all papers and the authors have been given the opportunity -

Clock Jitter Effects on the Performance of ADC Devices
Clock Jitter Effects on the Performance of ADC Devices Roberto J. Vega Luis Geraldo P. Meloni Universidade Estadual de Campinas - UNICAMP Universidade Estadual de Campinas - UNICAMP P.O. Box 05 - 13083-852 P.O. Box 05 - 13083-852 Campinas - SP - Brazil Campinas - SP - Brazil [email protected] [email protected] Karlo G. Lenzi Centro de Pesquisa e Desenvolvimento em Telecomunicac¸oes˜ - CPqD P.O. Box 05 - 13083-852 Campinas - SP - Brazil [email protected] Abstract— This paper aims to demonstrate the effect of jitter power near the full scale of the ADC, the noise power is on the performance of Analog-to-digital converters and how computed by all FFT bins except the DC bin value (it is it degrades the quality of the signal being sampled. If not common to exclude up to 8 bins after the DC zero-bin to carefully controlled, jitter effects on data acquisition may severely impacted the outcome of the sampling process. This analysis avoid any spectral leakage of the DC component). is of great importance for applications that demands a very This measure includes the effect of all types of noise, the good signal to noise ratio, such as high-performance wireless distortion and harmonics introduced by the converter. The rms standards, such as DTV, WiMAX and LTE. error is given by (1), as defined by IEEE standard [5], where Index Terms— ADC Performance, Jitter, Phase Noise, SNR. J is an exact integer multiple of fs=N: I. INTRODUCTION 1 s X = jX(k)j2 (1) With the advance of the technology and the migration of the rms N signal processing from analog to digital, the use of analog-to- k6=0;J;N−J digital converters (ADC) became essential. -

The Power Spectral Density of Phase Noise and Jitter: Theory, Data Analysis, and Experimental Results by Gil Engel
AN-1067 APPLICATION NOTE One Technology Way • P. O. Box 9106 • Norwood, MA 02062-9106, U.S.A. • Tel: 781.329.4700 • Fax: 781.461.3113 • www.analog.com The Power Spectral Density of Phase Noise and Jitter: Theory, Data Analysis, and Experimental Results by Gil Engel INTRODUCTION GENERAL DESCRIPTION Jitter on analog-to-digital and digital-to-analog converter sam- There are numerous techniques for generating clocks used in pling clocks presents a limit to the maximum signal-to-noise electronic equipment. Circuits include R-C feedback circuits, ratio that can be achieved (see Integrated Analog-to-Digital and timers, oscillators, and crystals and crystal oscillators. Depend- Digital-to-Analog Converters by van de Plassche in the References ing on circuit requirements, less expensive sources with higher section). In this application note, phase noise and jitter are defined. phase noise (jitter) may be acceptable. However, recent devices The power spectral density of phase noise and jitter is developed, demand better clock performance and, consequently, more time domain and frequency domain measurement techniques costly clock sources. Similar demands are placed on the spectral are described, limitations of laboratory equipment are explained, purity of signals sampled by converters, especially frequency and correction factors to these techniques are provided. The synthesizers used as sources in the testing of current higher theory presented is supported with experimental results applied performance converters. In the following section, definitions to a real world problem. of phase noise and jitter are presented. Then a mathematical derivation is developed relating phase noise and jitter to their frequency representation. -

LNCS 4523, Pp
中国科技论文在线 http://www.paper.edu.cn Jitter Distribution Evaluation and Suppression Method in UWB Systems Weihua Zhang, Hanbing Shen, Zhiquan Bai, and Kyung Sup Kwak UWB Wireless Communications Research Center (INHA UWB-ITRC), Inha University, 402-751, Incheon, Korea {zhweihua2000}@hotmail.com Abstract. For the extreme short duration of the baseband Impulse Ra- dio (IR) Ultra-Wideband (UWB) transmission waveforms, even small amounts of timing jitter can bring out considerable system performance degradation. Thus jitter performance evaluation and corresponding sup- pression methods are very important for UWB high rate transceiver. We analyzed the jitter distribution function in UWB systems, also a jitter suppression method is proposed by modification of template waveforms. Performance of our proposed waveforms and traditional template wave- forms are compared by analysis and simulation, and the results verify our jitter suppression method works.1 1 Introduction Ultra-wideband (UWB) is proposed as the primary candidate for the physical layer technology of next high speed short range wireless communication for per- sonal area networks (PAN) [1]. This radio technology is based on the radiation of waveforms formed by a sequence of very short, about nanosecond or below nanosecond, base band pulses [2]. The impulsive nature means that synchro- nization error (or jitter) influences the correlation of the received waveform and the template waveform generated in the receiver, thus deteriorates the system perform. Some past researches about this problem have been proposed: The effects of timing jitter on the bit error rate (BER) of UWB have been analyzed [3][4]. To suppressing this degradation system clock with higher stability is proposed which has the capability of precisely positioning the sub-nanosecond pulses with Gaussian distributed root-mean-squared (RMS) jitter of 10ps and within a 50ns time window [5]. -
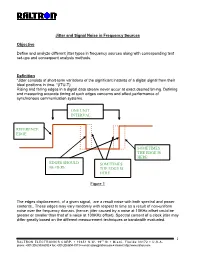
Jitter and Signal Noise in Frequency Sources
Jitter and Signal Noise in Frequency Sources Objective Define and analyze different jitter types in frequency sources along with corresponding test set-ups and consequent analysis methods. Definition “Jitter consists of short-term variations of the significant instants of a digital signal from their ideal positions in time. “(ITU-T) Rising and falling edges in a digital data stream never occur at exact desired timing. Defining and measuring accurate timing of such edges concerns and affect performance of synchronous communication systems. ONE UNIT INTERVAL REFERENCE EDGE SOMETIMES THE EDGE IS HERE EDGES SHOULD SOMETIMES BE HERE THE EDGE IS HERE Figure 1 The edges displacement, of a given signal, are a result noise with both spectral and power contents.. These edges may vary randomly with respect to time as a result of non-uniform noise over the frequency domain. (hence; jitter caused by a noise at 10KHz offset could be greater or smaller than that of a noise at 100KHz offset). Spectral content of a clock jitter may differ greatly based on the different measurement techniques or bandwidth evaluated. 1 RALTRON ELECTRONICS CORP. ! 10651 N.W. 19th St ! Miami, Florida 33172 ! U.S.A. phone: +001(305) 593-6033 ! fax: +001(305)594-3973 ! e-mail: [email protected] ! internet: http://www.raltron.com System Disruptions caused by Jitter Clock recovery mechanisms, in network elements, are used to sample the digital signal using the recovered bit clock. If the digital signal and the clock have identical jitter, the constant jitter error will not affect the sampling instant and therefore no bit errors will arise. -
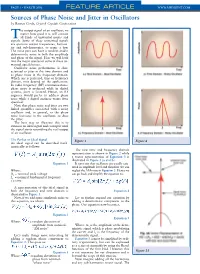
Sources of Phase Noise and Jitter in Oscillators by Ramon Cerda, Crystek Crystals Corporation
PAGE • MARCH 2006 FEATURE ARTICLE WWW.MPDIGEST.COM Sources of Phase Noise and Jitter in Oscillators by Ramon Cerda, Crystek Crystals Corporation he output signal of an oscillator, no matter how good it is, will contain Tall kinds of unwanted noises and signals. Some of these unwanted signals are spurious output frequencies, harmon- ics and sub-harmonics, to name a few. The noise part can have a random and/or deterministic noise in both the amplitude and phase of the signal. Here we will look into the major sources of some of these un- wanted signals/noises. Oscillator noise performance is char- acterized as jitter in the time domain and as phase noise in the frequency domain. Which one is preferred, time or frequency domain, may depend on the application. In radio frequency (RF) communications, phase noise is preferred while in digital systems, jitter is favored. Hence, an RF engineer would prefer to address phase noise while a digital engineer wants jitter specified. Note that phase noise and jitter are two linked quantities associated with a noisy oscillator and, in general, as the phase noise increases in the oscillator, so does the jitter. The best way to illustrate this is to examine an ideal signal and corrupt it until the signal starts resembling the real output of an oscillator. The Perfect or Ideal Signal Figure 1 Figure 2 An ideal signal can be described math- ematically as follows: The new time and frequency domain representation is shown in Figure 2 while a vector representation of Equation 3 is illustrated in Figure 3 (a and b.) Equation 1 It turns out that oscillators are usually satu- rated in amplitude level and therefore we can Where: neglect the AM noise in Equation 3. -

Understanding and Characterizing Timing Jitter ––
Understanding and Characterizing Timing Jitter –– PRIMER Primer Table of Contents Introduction ....................................................................3 Section 4: Jitter Separation .........................................13 4.1 Motivations for Decomposing Jitter ...........................13 Section 1: The Consequences of Jitter .........................3 4.2 The Jitter Model ........................................................13 Computer Bus Design .......................................................3 4.2.1 Random Jitter ..................................................14 Serial Data Link .................................................................4 4.2.2 Deterministic Jitter ............................................15 Section 2: Just What Is Jitter? .......................................4 4.2.3 Periodic Jitter ...................................................15 2.1 Defining Short-Term: Jitter vs. Wander ........................4 4.2.4 Data-Dependent Jitter ......................................16 2.2 Defining Significant Instants: The Reference Level .......5 4.2.5 Duty-Cycle Dependent Jitter .............................16 2.3 Defining Ideal Positions: Clock Recovery .....................5 4.2.6 Bounded Uncorrelated Jitter .............................17 2.4 Period Jitter, Cycle-Cycle Jitter, and TIE ......................6 4.2.7 Sub-Rate Jitter .................................................17 4.3 Putting It All Together ................................................18 Section 3: Jitter Measurement