Willow Identification Guide (DSE Vic)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Weeping Willow Salix Babylonica
Weeping willow Salix babylonica Description Introduced to North America as an ornamental. Habit Perennial tree to 40 ft tall; rounded crown; long, hanging branches; grayish-brown, irregularly furrowed bark. Leaves Alternate, simple, very narrowly lance-shaped, finely serrated margin, yellow-green above and milky green below, 3-6 inches in length, 3/8 to 1/2 inch in width. Stems Very slender; smooth; olive-green to pale yellowish brown; hanging or drooping for long distances; almost rope-like; buds are small, appressed and covered by a single, cap-like Source: MISIN. 2021. Midwest Invasive Species Information Network. Michigan State University - Applied Spatial Ecology and Technical Services Laboratory. Available online at https://www.misin.msu.edu/facts/detail.php?id=148. scale; terminal buds lacking. Flowers Dioecious, males and females appear as upright catkins and are quite fuzzy, 1 inch long, appearing before or with the leaves. Fruits and Seeds A 1 in long cluster of valve-like capsules, light brown in color, contains many fine, cottony seeds; ripen in late May to early June. Habitat Native to Asia. Reproduction By herbaceous stem cuttings, woody stem cuttings or softwood cuttings. Similar White Willow (Salix alba); Black Willow (Salix nigra); Corkscrew Willow (Salix matsudana Koidzumi ). Monitoring and Rapid Response Hand pull small seedlings; use machinery to remove larger trees and root systems in dry areas; effectively controlled using any of several readily available general use herbicides such as glyphosate. Credits The information provided in this factsheet was gathered from the USDA PLANTS Database and Virginia Tech Department of Forest Resources and Environmental Conservation VTree. -
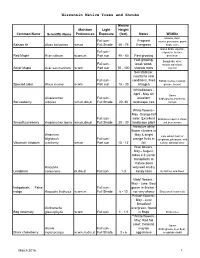
Wisconsin Native Trees and Shrubs
Wisconsin Native Trees and Shrubs Mature Moisture Light Height Common Name Scientific Name Preferences Exposure (feet) Notes Wildlife Grouse, deer, Full sun - Fragrant moose,porcupine, game Balsam fir Abies balsamea wm,m Full Shade 40 - 75 Evergreen birds, mice Game birds, squirrel, Full sun - chipmunk, beaver, Red Maple Acer rubrum w,wm,m Part sun 40 - 60 Fast growing deer,bear Fast growing, Songbirds, deer, Full sun - weak wood, racoon,waterfowl, Silver Maple Acer saccharinum w,wm Part sun 75 - 100 shallow roots squirrel Soil stablizer, neutral to acid Full sun - conditions, fixes Rabbit,moose,muskrat, Specled alder Alnus incana w,wm Part sun 15 - 30 nitrogen grouse, beaver Whiteflowers - April - May An Game Amelanchier Full sun - excellent birds,grouse,skunk,fox, Serviceberry arborea wm,m,dm,d Full Shade 20 -30 landscape tree racoon White flowers - May Orange fall Full sun - color Excellent Birds,bear,squirrel,chipm Smooth juneberry Amelanchier laevis wm,m,dm,d Full Shade 20 - 30 landscape plant unk,deer,moose Attractive white flower clusters in American May & bright Late winter food for Highbush Full sun - orange fruits in songbirds, pheasant, wild Viburnum trilobum cranberry wm,m Part sun 10 - 13' fall turkey, whitetail deer Blue flowers, May - August; takes 2-3 yrs for transplants to mature;does Amorpha very well on dry Leadplant canescens m,dm,d Full sun 1-3 sandy sites Butterflies and Bees Violet flowers - May - June Best Indigobush; False Full sun - grown in thicket - indigo Amorpha fruticosa w,wm,m Full Shade 6 - 12 not very -

Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Cultivated Willows Would Not Be Appropriate Without Mention of the ‘WEEPING WILLOW’
Alexander Robertson Notes on willows cultivated in Scotland and a few sketches of willows sampled from my clonal collection HISTORICAL NOTES Since the knowledge of willows is of great antiquity, it is with the ancient Greeks and Romans we shall begin, for among these people numerous written records remain. The growth habit, ecology, cultivation and utilization of willows was well— understood by Theophrastus, Ovid, Herodotus, Pliny and Dioscorides. Virgil was also quite familiar with willow, e.g. Damoetas complains that: “Galatea, saucy girl, pelts me with apples and then runs off to the willows”. ECLOGIJE III and of foraging bees: “Far and wide they feed on arbutus, pale-green willows, on cassia and ruddy crocus .. .“ GEORGICS IV Theophrastus of Eresos (370—285 B.C.) discussed many aspects of willows throughout his Enquiry into Plants including habitats, wood quality, coppicing and a variety of uses. Willows, according to Theophrastus are lovers of wet places and marshes. But he also notes certain amphibious traits of willows growing in mountains and plains. To Theophrastus they appeared to possess no fruits and quite adequately reproduced themselves from roots, were tolerant to flooding and frequent coppicing. “Even willows grow old and when they are cut, no matter at what height, they shoot up again.” He described the wood as cold, tough, light and resilient—qualities which made it useful for a variety of purposes, especially shields. Such were the diverse virtues of willow that he suggested introducing it for plant husbandry. Theophrastus noted there were many different kinds of willows; three of the best known being black willow (Salix fragilis), white willow (S. -

Salix L.) in the European Alps
diversity Review The Evolutionary History, Diversity, and Ecology of Willows (Salix L.) in the European Alps Natascha D. Wagner 1 , Li He 2 and Elvira Hörandl 1,* 1 Department of Systematics, Biodiversity and Evolution of Plants (with Herbarium), University of Goettingen, Untere Karspüle 2, 37073 Göttingen, Germany; [email protected] 2 College of Forestry, Fujian Agriculture and Forestry University, Fuzhou 350002, China; [email protected] * Correspondence: [email protected] Abstract: The genus Salix (willows), with 33 species, represents the most diverse genus of woody plants in the European Alps. Many species dominate subalpine and alpine types of vegetation. Despite a long history of research on willows, the evolutionary and ecological factors for this species richness are poorly known. Here we will review recent progress in research on phylogenetic relation- ships, evolution, ecology, and speciation in alpine willows. Phylogenomic reconstructions suggest multiple colonization of the Alps, probably from the late Miocene onward, and reject hypotheses of a single radiation. Relatives occur in the Arctic and in temperate Eurasia. Most species are widespread in the European mountain systems or in the European lowlands. Within the Alps, species differ eco- logically according to different elevational zones and habitat preferences. Homoploid hybridization is a frequent process in willows and happens mostly after climatic fluctuations and secondary contact. Breakdown of the ecological crossing barriers of species is followed by introgressive hybridization. Polyploidy is an important speciation mechanism, as 40% of species are polyploid, including the four endemic species of the Alps. Phylogenomic data suggest an allopolyploid origin for all taxa analyzed Citation: Wagner, N.D.; He, L.; so far. -

Responses of Black Willow (Salix Nigra) Cuttings to Simulated Herbivory and flooding
Acta Oecologica 28 (2005) 173–180 www.elsevier.com/locate/actoec Original article Responses of black willow (Salix nigra) cuttings to simulated herbivory and flooding Shuwen Li a,*, Lili T. Martin a, S. Reza Pezeshki a, F. Douglas Shields Jr. b a Department of Biology, The University of Memphis, Memphis, TN 38152, USA b USDA-ARS National Sedimentation Laboratory, P.O. Box 1157, Oxford, MS 38655, USA Received 7 January 2004; accepted 31 March 2005 Available online 04 May 2005 Abstract Herbivory and flooding influence plant species composition and diversity in many wetland ecosystems. Black willow (Salix nigra) natu- rally occurs in floodplains and riparian zones of the southeastern United States. Cuttings from this species are used as a bioengineering tool for streambank stabilization and habitat rehabilitation. The present study was conducted to evaluate the photosynthetic and growth responses of black willow to simulated herbivory and flooding. Potted cuttings were subjected to three levels of single-event herbivory: no herbivory (control), light herbivory, and heavy herbivory; and three levels of flooding conditions: no flooding (control), continuous flooding, and peri- odic flooding. Results indicated that elevated stomatal conductance partially contributed to the increased net photosynthesis noted under both levels of herbivory on day 30. However, chlorophyll content was not responsible for the observed compensatory photosynthesis. Cuttings subjected to heavy herbivory accumulated the lowest biomass even though they had the highest height growth by the conclusion of the experiment. In addition, a reduction in root/shoot ratio was noted for plants subjected to continuous flooding with no herbivory. However, continuously flooded, lightly clipped plants allocated more resources to roots than shoots. -

Salix × Meyeriana (= Salix Pentandra × S
Phytotaxa 22: 57–60 (2011) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Correspondence PHYTOTAXA Copyright © 2011 Magnolia Press ISSN 1179-3163 (online edition) Salix × meyeriana (= Salix pentandra × S. euxina)―a forgotten willow in Eastern North America ALEXEY G. ZINOVJEV 9 Madison Ave., Randolph, MA 02368, USA; E-mail: [email protected] Salix pentandra L. is a boreal species native to Europe and western Siberia. In North America it is considered to have been introduced to about half of the US states (Argus 2007, 2010). In Massachusetts it is reported from nine of the fourteen counties (Sorrie & Somers 1999). Even though this plant may have been introduced to the US and Canada, its naturalization in North America appears to be quite improbable. Unlike willows from the related section Salix, in S. pentandra twigs are not easily broken off and their ability to root is very low, 0–15% (Belyaeva et al. 2006). It is possible to propagate S. pentandra from softwood cuttings (Belyaeva et al. 2006) and it can be cultivated in botanical gardens, however, vegetative reproduction of this species by natural means seems less likely. In North America this willow is known only by female (pistillate) plants (Argus 2010), so for this species, although setting fruit, sexual reproduction should be excluded. It is difficult to imagine that, under these circumstances, it could escape from cultivation. Therefore, most of the records for this willow in North America should be considered as collections from cultivated plants or misidentifications (Zinovjev 2008–2010). Salix pentandra is known to hybridize with willows of the related section Salix. -

3.2.2.11. Familia Salicaceae (Incluyendo a Flacourtiaceae) 3.2.2.11.A
97 3.2.2.11. Familia Salicaceae (incluyendo a Flacourtiaceae) 3.2.2.11.a. Características ¾ Porte: arbustos o árboles. ¾ Hojas: alternas, simples, con estípulas, en general caducas. ¾ Flores: pequeñas, imperfectas, diclino-dioicas, en amentos erguidos o péndulos. En Azara, Cassearia, Banara, Xylosma pequeñas, solitarias, axilares o en cimas, perfectas o imperfectas, hipóginas, raro períginas o epíginas. ¾ Perianto: aperiantadas, protegidas por una bráctea, con un cáliz vestigial, en Salix se reduce a nectarios. En Azara, Cassearia, Banara, Xylosma cáliz con 3-15 sépalos libres; corola, 3-15 pétalos, disco nectarífero intrastaminal o extraestaminal. ¾ Estambres: 2-varios. En Azara, Cassearia, Banara, Xylosma 4-∞, libres, anteras ditecas. ¾ Gineceo: ovario súpero, 2-10 carpelos unidos, unilocular, pluriovulado, óvulos 1-∞, parietales, estilos libres, parcialmente soldados o estilo único, estigmas. ¾ Fruto: cápsula dehiscente conteniendo semillas lanosas. En Azara, Cassearia, Banara, Xylosma baya, cápsula loculicida o drupa. ¾ Semillas: con pelos, sin endosperma y con embrión recto. Azara, Cassearia, Banara, Xylosma semillas ariladas. Flor estaminada, flor pistilada, brácteas y nectario de la flor estaminada de Salix caroliniana Flor estaminada y flor pistilada de Azara microphylla (Dibujos adaptados de Boelcke y Vizinis, 1987 por Daniel Cian) Diversidad Vegetal Facultad de Ciencias Exactas y Naturales y Agrimensura (UNNE) EUDICOTILEDONEAS ESCENCIALES-Clado Rosides-Eurosides I-Malpighiales: Salicaceae (inc. Flacourtiaceae) 98 3.2.2.11.b. Biología floral y/o Fenología La polinización puede ser anemófila, en Populus, o por insectos atraídos por el néctar, que producen los nectarios, ubicados en la base de la flor. Especies del género Salix son polinizadas por abejas melíferas. En las especies entomófilas los órganos nectaríferos son foliares, residuos del perianto que desapareció (Vogel, com. -

Salix Nigra Marsh
Salix nigra Marsh Salix nigra Marsh. Black Willow Salicaceae -- Willow family J. A. Pitcher and J. S. McKnight Black willow (Salix nigra) is the largest and the only commercially important willow of about 90 species native to North America. It is more distinctly a tree throughout its range than any other native willow; 27 species attain tree size in only part of their range (3). Other names sometimes used are swamp willow, Goodding willow, southwestern black willow, Dudley willow, and sauz (Spanish). This short-lived, fast-growing tree reaches its maximum size and development in the lower Mississippi River Valley and bottom lands of the Gulf Coastal Plain (4). Stringent requirements of seed germination and seedling establishment limit black willow to wet soils near water courses (5), especially floodplains, where it often grows in pure stands. Black willow is used for a variety of wooden products and the tree, with its dense root system, is excellent for stabilizing eroding lands. Habitat Native Range Black willow is found throughout the Eastern United States and adjacent parts of Canada and Mexico. The range extends from southern New Brunswick and central Maine west in Quebec, southern Ontario, and central Michigan to southeastern Minnesota; south and west to the Rio Grande just below its confluence with the Pecos River; and east along the gulf coast, through the Florida panhandle and southern Georgia. Some authorities consider Salix gooddingii as a variety of S. nigra, which extends the range to the Western United States (3,9). http://www.na.fs.fed.us/spfo/pubs/silvics_manual/volume_2/salix/nigra.htm (1 of 9)1/4/2009 4:08:21 PM Salix nigra Marsh -The native range of black willow. -

Name Clarification: Salix Babylonica L. Vs. S. Matsudana Koidz
Name clarification: Salix babylonica L. vs. S. matsudana Koidz. Fact Sheet No 4 July 2018 Yulia A. Kuzovkina1 and Irina V. Belyaeva2 1Department of Plant Science and Landscape Architecture, Unit-4067, University of Connecticut, Storrs, CT 06269-4067, USA 2Royal Botanic Gardens, Kew, Richmond, TW9 3AE, UK The two binominals – S. babylonica L. vs. S. matsudana Koidz. – are frequently used interchangeably in references, scientific publications and catalogs. This discrepancy stems from a difference of opinion on the circumscription or the relationships of the taxa among taxonomists who fall into two camps. In some cases S. matsudana is considered to be the synonym of S. babylonica, and in other cases, these two names denote two different species. Salix babylonica ‘Tortuosa’, dragon’s claw willow or curly willow, an ornamental cultivar with contorted stems. Photo courtesy of M. Dodge, Vermont Willow Nursery. 1 Salix matsudana was described by Koidzumi (1915) referring to the non-weeping taxon from China. This segregation was followed by Rehder (1927, 1940, 1949) who treated “less weeping selections” from China as S. matsudana. Fang et al. (1999), Ohashi (2001) also remarked that the unique characteristics of S. matsudana merit recognition as a distinct species apart from S. babylonica. Another group of botanists, including Skvortsov (1999), Santamour and McArdle (1988) believe that there is little evidence to substantiate the notion of the separation of the two species, and that it is biologically more sensible to regard S. matsudana as synonymous with S. babylonica. In their recent publication Ohashi and Yonekura (2015) regarded S. matsudana as conspecific with S. babylonica and treated it newly as a variety, S .