AN AUTECOLOGICAL STUDY of SALIX NIGRA, ESPECIALLY FACTORS TJ:JAT MIGHT LIMIT ITS DISTRIBUTION Major Field: Botany and Plant Pathology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -
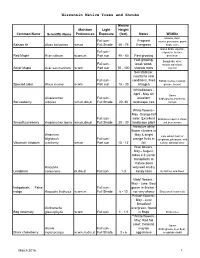
Wisconsin Native Trees and Shrubs
Wisconsin Native Trees and Shrubs Mature Moisture Light Height Common Name Scientific Name Preferences Exposure (feet) Notes Wildlife Grouse, deer, Full sun - Fragrant moose,porcupine, game Balsam fir Abies balsamea wm,m Full Shade 40 - 75 Evergreen birds, mice Game birds, squirrel, Full sun - chipmunk, beaver, Red Maple Acer rubrum w,wm,m Part sun 40 - 60 Fast growing deer,bear Fast growing, Songbirds, deer, Full sun - weak wood, racoon,waterfowl, Silver Maple Acer saccharinum w,wm Part sun 75 - 100 shallow roots squirrel Soil stablizer, neutral to acid Full sun - conditions, fixes Rabbit,moose,muskrat, Specled alder Alnus incana w,wm Part sun 15 - 30 nitrogen grouse, beaver Whiteflowers - April - May An Game Amelanchier Full sun - excellent birds,grouse,skunk,fox, Serviceberry arborea wm,m,dm,d Full Shade 20 -30 landscape tree racoon White flowers - May Orange fall Full sun - color Excellent Birds,bear,squirrel,chipm Smooth juneberry Amelanchier laevis wm,m,dm,d Full Shade 20 - 30 landscape plant unk,deer,moose Attractive white flower clusters in American May & bright Late winter food for Highbush Full sun - orange fruits in songbirds, pheasant, wild Viburnum trilobum cranberry wm,m Part sun 10 - 13' fall turkey, whitetail deer Blue flowers, May - August; takes 2-3 yrs for transplants to mature;does Amorpha very well on dry Leadplant canescens m,dm,d Full sun 1-3 sandy sites Butterflies and Bees Violet flowers - May - June Best Indigobush; False Full sun - grown in thicket - indigo Amorpha fruticosa w,wm,m Full Shade 6 - 12 not very -

Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Responses of Black Willow (Salix Nigra) Cuttings to Simulated Herbivory and flooding
Acta Oecologica 28 (2005) 173–180 www.elsevier.com/locate/actoec Original article Responses of black willow (Salix nigra) cuttings to simulated herbivory and flooding Shuwen Li a,*, Lili T. Martin a, S. Reza Pezeshki a, F. Douglas Shields Jr. b a Department of Biology, The University of Memphis, Memphis, TN 38152, USA b USDA-ARS National Sedimentation Laboratory, P.O. Box 1157, Oxford, MS 38655, USA Received 7 January 2004; accepted 31 March 2005 Available online 04 May 2005 Abstract Herbivory and flooding influence plant species composition and diversity in many wetland ecosystems. Black willow (Salix nigra) natu- rally occurs in floodplains and riparian zones of the southeastern United States. Cuttings from this species are used as a bioengineering tool for streambank stabilization and habitat rehabilitation. The present study was conducted to evaluate the photosynthetic and growth responses of black willow to simulated herbivory and flooding. Potted cuttings were subjected to three levels of single-event herbivory: no herbivory (control), light herbivory, and heavy herbivory; and three levels of flooding conditions: no flooding (control), continuous flooding, and peri- odic flooding. Results indicated that elevated stomatal conductance partially contributed to the increased net photosynthesis noted under both levels of herbivory on day 30. However, chlorophyll content was not responsible for the observed compensatory photosynthesis. Cuttings subjected to heavy herbivory accumulated the lowest biomass even though they had the highest height growth by the conclusion of the experiment. In addition, a reduction in root/shoot ratio was noted for plants subjected to continuous flooding with no herbivory. However, continuously flooded, lightly clipped plants allocated more resources to roots than shoots. -

Salix Nigra Marsh
Salix nigra Marsh Salix nigra Marsh. Black Willow Salicaceae -- Willow family J. A. Pitcher and J. S. McKnight Black willow (Salix nigra) is the largest and the only commercially important willow of about 90 species native to North America. It is more distinctly a tree throughout its range than any other native willow; 27 species attain tree size in only part of their range (3). Other names sometimes used are swamp willow, Goodding willow, southwestern black willow, Dudley willow, and sauz (Spanish). This short-lived, fast-growing tree reaches its maximum size and development in the lower Mississippi River Valley and bottom lands of the Gulf Coastal Plain (4). Stringent requirements of seed germination and seedling establishment limit black willow to wet soils near water courses (5), especially floodplains, where it often grows in pure stands. Black willow is used for a variety of wooden products and the tree, with its dense root system, is excellent for stabilizing eroding lands. Habitat Native Range Black willow is found throughout the Eastern United States and adjacent parts of Canada and Mexico. The range extends from southern New Brunswick and central Maine west in Quebec, southern Ontario, and central Michigan to southeastern Minnesota; south and west to the Rio Grande just below its confluence with the Pecos River; and east along the gulf coast, through the Florida panhandle and southern Georgia. Some authorities consider Salix gooddingii as a variety of S. nigra, which extends the range to the Western United States (3,9). http://www.na.fs.fed.us/spfo/pubs/silvics_manual/volume_2/salix/nigra.htm (1 of 9)1/4/2009 4:08:21 PM Salix nigra Marsh -The native range of black willow. -
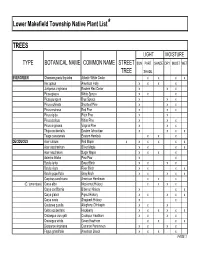
Native Plant List Trees.XLS
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v. -

Vegetation Community Monitoring at Ocmulgee National Monument, 2011
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Vegetation Community Monitoring at Ocmulgee National Monument, 2011 Natural Resource Data Series NPS/SECN/NRDS—2014/702 ON THE COVER Duck potato (Sagittaria latifolia) at Ocmulgee National Monument. Photograph by: Sarah C. Heath, SECN Botanist. Vegetation Community Monitoring at Ocmulgee National Monument, 2011 Natural Resource Data Series NPS/SECN/NRDS—2014/702 Sarah Corbett Heath1 Michael W. Byrne2 1USDI National Park Service Southeast Coast Inventory and Monitoring Network Cumberland Island National Seashore 101 Wheeler Street Saint Marys, Georgia 31558 2USDI National Park Service Southeast Coast Inventory and Monitoring Network 135 Phoenix Road Athens, Georgia 30605 September 2014 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Data Series is intended for the timely release of basic data sets and data summaries. Care has been taken to assure accuracy of raw data values, but a thorough analysis and interpretation of the data has not been completed. Consequently, the initial analyses of data in this report are provisional and subject to change. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

APPENDIX B Biological Resources Vegetation Species Lists
Feasibility Investigation Report Restoration of Hydrology along Mobile Bay Causeway December 2015 APPENDIX B Biological Resources Vegetation Species Lists Weston Solutions, Inc. Choccolatta Bay, June 2014 ORDER SALVINIALES SALVINIACEAE (FLOATING FERN FAMILY) Azolla caroliniana Willdenow —EASTERN MOSQUITO FERN, CAROLINA MOSQUITO FERN Salvinia minima Baker —WATER-SPANGLES, COMMON SALVINIA† ORDER ALISMATALES ARACEAE (ARUM FAMILY) Lemna obscura (Austin) Daubs —LITTLE DUCKWEED Spirodela polyrrhiza (Linnaeus) Schleiden —GREATER DUCKWEED ALISMATACEAE (MUD PLANTAIN FAMILY) Sagittaria lancifolia Linnaeus —BULLTONGUE ARROWHEAD HYDROCHARITACEAE (FROG’S-BIT FAMILY) Najas guadalupensis (Sprengel) Magnus —COMMON NAIAD, SOUTHERN NAIAD ORDER ASPARAGALES AMARYLLIDACEAE (AMARYLLIS FAMILY) Allium canadense Linnaeus var. canadense —WILD ONION ORDER COMMELINALES COMMELINACEAE (SPIDERWORT FAMILY) Commelina diffusa Burman f. —SPREADING DAYFLOWER, CLIMBING DAYFLOWER† PONTEDERIACEAE (PICKERELWEED FAMILY) Eichhornia crassipes (Martius) Solms —WATER HYACINTH† Pontederia cordata Linnaeus —PICKEREL WEED ORDER POALES TYPHACEAE (CATTAIL FAMILY) Typha domingensis Persoon —SOUTHERN CATTAIL JUNCACEAE (RUSH FAMILY) Juncus marginatus Rostkovius —GRASSLEAF RUSH † = non-native naturalized or invasive taxa Choccolatta Bay, June 2014 CYPERACEAE (SEDGE FAMILY) Cyperus esculentus Linnaeus —YELLOW NUTGRASS, CHUFA FLATSEDGE† Cyperus strigosus Linnaeus —STRAW-COLOR FLATSEDGE Schoenoplectus deltarum (Schuyler) Soják —DELTA BULRUSH Schoenoplectus tabernaemontani (C.C. Gmelin) Palla -
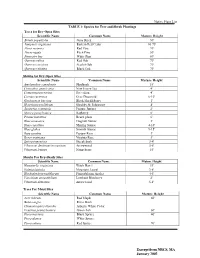
Native Plant List TABLE 1: Species for Tree and Shrub Plantings Trees For
Native Plant List TABLE 1: Species for Tree and Shrub Plantings Trees for Dry-Open Sites Scientific Name Common Name Mature Height Betula populifolia Gray Birch 30' Juniperis virginiana Eastern Red Cedar 10-75' Pinus resinosa Red Pine 70' Pin us rigida Pitch Pine 50' Pinus stro bus White Pine 80' Quercus rubra Red Oak 70' Quercus coccinea Scarlet Oak 70' Quercus velutina Black Oak 70' Shrubs for Dry-Open Sites Scientific Name 'Common Name Mature Height Amelanchier canadensis Shadbush 15' Ceanothus americanus New Jersey Tea 4' Comptonia peregrina Sweetfern 4' Cornus racemosa Gray Dogwood 6-10' Gaylussacia baccata Black Huckleberry l' Hypericum prolificum Shrubby St. Johnswort 4' Juniperus communis Pasture Juniper 2' Myrica pensylvanica Bayberry 6' Prunus maritima Beach plum 6' Rhus aromatica Fragrant Sumac 3' Rhus copallina Shining Sumac 4-10' Rhus glabra Smooth Sumac 9-15' Rosa carolina Pasture Rose 3' Rosa virginiana Virginia Rose 3' Spirea tomentosa Steeplebush 3-4' Viburnum dentatum/recognitum Arrowwood 5-8' Viburnum lentago Nannyberry 15' Shrubs For Dry-Shady Sites Scientific Name Common Name Mature Height Hamamelis wrginiana Witch Hazel 15' Kalmia latifolia Mountain Laurel 3-8' Rhododendron nudiflorum Pinxterbloom Azalea 4-6' Vaccinium angustifolium Lowbush Blueberry 2' Viburnum dentatum Arrowwood 5-8' Trees For Moist Sites Scientific Name Common Name Mature Height Acer rubrum Red Maple 60' Betula nigra River Birch Chamaecyparis thyoides Atlantic White Cedar Fraxinus pennsylvanica Green Ash 60' Picea mariana Black Spruce 40' Picea -

BP―Willow Bark Extract Natural Source of Beta Hydroxy Acid TECHNICAL BULLETIN
BP―Willow Bark Extract Natural Source of Beta Hydroxy Acid TECHNICAL BULLETIN INCI Name: Water & Salix Nigra (Willow) Bark Our unique extraction and standardization Extract (measured and confirmed on every batch Key Benefits: through UV Vis Spectroscopy) assures a consistent • Naturally-derived ingredient standardized extract with a range of 9% to 12% • Gentle yet effective salicylic acid in every batch. BP–Willow Bark • Perfect for oily skin, fights acne Extract is a more gentle and effective β-hydroxy • Reduces inflammation acid, without the risk of irritation typically caused • Opens clogged pores, loosens by synthetically produced salicylic acid. blackheads Background Typical Properties For centuries, Willow Bark Extract has been used Colorless to light amber Appearance @ 25°C in cosmetics and personal care products for its liquid astringent, anti-inflammatory, antiseptic, soothing, Odor Characteristic and conditioning properties. Folklore states that it Color (Gardner) 4.0 Maximum has been also used as a treatment for headaches pH (Direct @ 25°C) 4.0 – 6.5 and other ailments due to its aspirin-like properties. NVM (1g-1hr-105°C) 25.0% Minimum Willow Bark Extract contains salicylic acid, which is Salicylic Acid (UV) 9.8 - 11.5% a natural exfoliant and a Beta-Hydroxy Acid (BHA) Identification (FT-IR Spectra) To Match Standard that is used in many acne treatments because of Microbial Content <100 opg, no pathogens its ability to help skin shed dead cells and clear pores; it can also stimulate new cell formation. Recommended Use Level -

Maine Tree Species Fact Sheet
Maine Tree Species Fact Sheet Common Name: Black Willow (Swamp Willow, Dudley Willow) Botanical Name: Salix nigra y/commontr/images/BlackWillow.gif Tree Type: Deciduous Physical Description: Growth Habit: Black willow is the largest native willow. It is a short-lived and fast-growing tree. The bark is thick and rough with wide ridges covered by thick scales. It varies in color from light to dark brown. The twigs are slender, smooth, somewhat drooping, very brittle at the base and reddish-brown in color. Fallen twigs may take root and grow. The alternate and simple http://www.dcnr.state.pa.us/Forestr leaves are very narrow, sometimes sickle-shaped, finely toothed and 3-6 inches long. They are dark green in color above and pale green below Height: The black willow grows to a height of 45-65 feet. Shape: Black willow often divides into several crooked, medium-sized trunks close to the ground. The stout, upright spreading branches give the tree a broad, irregular outline. Fruit/Seed Description/Dispersal Methods: The flowers are dioecious and appear in late June. The many-flowered catkins usually appear at the time of, or immediately after, the tree has leafed out. The fruit is a smooth capsule, about 1/8-inch long, occurring in large numbers on drooping tassels. They ripen in the spring and are reddish-brown in color. Seeds appear within the capsule and are covered with a dense tuft of long silky hairs. When the seeds fall, the hairs act as wings to carry them very long distances. The seeds are widely disseminated by wind and water. -

Vegetation Community Monitoring at Congaree National Park: 2014 Data Summary
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Vegetation Community Monitoring at Congaree National Park 2014 Data Summary Natural Resource Data Series NPS/SECN/NRDS—2016/1016 ON THIS PAGE Tiny, bright yellow blossoms of Hypoxis hirsuta grace the forest floor at Congaree National Park. Photograph courtesy of Sarah C. Heath, Southeast Coast Network. ON THE COVER Spiraling compound leaf of green dragon (Arisaema dracontium) at Congaree National Park. Photograph courtesy of Sarah C. Heath, Southeast Coast Network Vegetation Community Monitoring at Congaree National Park 2014 Data Summary Natural Resource Data Series NPS/SECN/NRDS—2016/1016 Sarah Corbett Heath1 and Michael W. Byrne2 1National Park Service Southeast Coast Inventory and Monitoring Network Cumberland Island National Seashore 101 Wheeler Street Saint Marys, GA 31558 2National Park Service Southeast Coast Inventory and Monitoring Network 135 Phoenix Drive Athens, GA 30605 May 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Data Series is intended for the timely release of basic data sets and data summaries. Care has been taken to assure accuracy of raw data values, but a thorough analysis and interpretation of the data has not been completed.