Diseases of Poplars and Willows*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Populusspp. Family: Salicaceae Aspen
Populus spp. Family: Salicaceae Aspen Aspen (the genus Populus) is composed of 35 species which contain the cottonwoods and poplars. Species in this group are native to Eurasia/north Africa [25], Central America [2] and North America [8]. All species look alike microscopically. The word populus is the classical Latin name for the poplar tree. Populus grandidentata-American aspen, aspen, bigtooth aspen, Canadian poplar, large poplar, largetooth aspen, large-toothed poplar, poplar, white poplar Populus tremuloides-American aspen, American poplar, aspen, aspen poplar, golden aspen, golden trembling aspen, leaf aspen, mountain aspen, poplar, popple, quaking asp, quaking aspen, quiver-leaf, trembling aspen, trembling poplar, Vancouver aspen, white poplar Distribution Quaking aspen ranges from Alaska through Canada and into the northeastern and western United States. In North America, it occurs as far south as central Mexico at elevations where moisture is adequate and summers are sufficiently cool. The more restricted range of bigtooth aspen includes southern Canada and the northern United States, from the Atlantic coast west to the prairie. The Tree Aspens can reproduce sexually, yielding seeds, or asexually, producing suckers (clones) from their root system. In some cases, a stand could then be composed of only one individual, genetically, and could be many years old and cover 100 acres (40 hectares) or more. Most aspen stands are a mosaic of several clones. Aspen can reach heights of 120 ft (48 m), with a diameter of 4 ft (1.6 m). Aspen trunks can be quite cylindrical, with little taper and few limbs for most of their length. They also can be very crooked or contorted, due to genetic variability. -

Towards an Understanding of the Evolution of Violaceae from an Anatomical and Morphological Perspective Saul Ernesto Hoyos University of Missouri-St
University of Missouri, St. Louis IRL @ UMSL Theses Graduate Works 8-7-2011 Towards an understanding of the evolution of Violaceae from an anatomical and morphological perspective Saul Ernesto Hoyos University of Missouri-St. Louis, [email protected] Follow this and additional works at: http://irl.umsl.edu/thesis Recommended Citation Hoyos, Saul Ernesto, "Towards an understanding of the evolution of Violaceae from an anatomical and morphological perspective" (2011). Theses. 50. http://irl.umsl.edu/thesis/50 This Thesis is brought to you for free and open access by the Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Theses by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. Saul E. Hoyos Gomez MSc. Ecology, Evolution and Systematics, University of Missouri-Saint Louis, 2011 Thesis Submitted to The Graduate School at the University of Missouri – St. Louis in partial fulfillment of the requirements for the degree Master of Science July 2011 Advisory Committee Peter Stevens, Ph.D. Chairperson Peter Jorgensen, Ph.D. Richard Keating, Ph.D. TOWARDS AN UNDERSTANDING OF THE BASAL EVOLUTION OF VIOLACEAE FROM AN ANATOMICAL AND MORPHOLOGICAL PERSPECTIVE Saul Hoyos Introduction The violet family, Violaceae, are predominantly tropical and contains 23 genera and upwards of 900 species (Feng 2005, Tukuoka 2008, Wahlert and Ballard 2010 in press). The family is monophyletic (Feng 2005, Tukuoka 2008, Wahlert & Ballard 2010 in press), even though phylogenetic relationships within Violaceae are still unclear (Feng 2005, Tukuoka 2008). The family embrace a great diversity of vegetative and floral morphologies. Members are herbs, lianas or trees, with flowers ranging from strongly spurred to unspurred. -

Salicaceae Cottonwood Cottonwood (The Genus Populus) Is Composed of 35 Species Which Contain the Aspens and Poplars
Populus spp. Family: Salicaceae Cottonwood Cottonwood (the genus Populus) is composed of 35 species which contain the aspens and poplars. Species in this group are native to Eurasia/north Africa [25], Central America [2] and North America [8]. All species look alike microscopically. The word populus is the classical Latin name for the poplar tree. Populus angustifolia-balsam, bitter cottonwood, black cottonwood, lanceleaf cottonwood, mountain cottonwood, narrowleaf cottonwood, narrow leaved poplar, Rydberg cottonwood, smoothbark cottonwood, willow cottonwood, willowleaf cottonwood Populus balsamifera-balm, balm of Gilead, balm of Gilead poplar, balm cottonwood, balsam, balsam cottonwood, balsam poplar, bam, black balsam poplar, black cottonwood, black poplar, California poplar, Canadian balsam poplar, Canadian poplar, cottonwax, hackmatack, hairy balm of Gilead, heartleaf balsam poplar, northern black cottonwood, Ontario poplar, tacamahac, tacamahac poplar, toughbark poplar, western balsam poplar Populus deltoides*-aspen cottonwood, big cottonwood, Carolina poplar, cotton tree, eastern cottonwood, eastern poplar, fremont cottonwood, great plains cottonwood, Missourian poplar, necklace poplar, northern fremont cottonwood, palmer cottonwood, plains cottonwood, Rio Grande cottonwood, river cottonwood, river poplar, southern cottonwood, Tennessee poplar, Texas cottonwood, valley cottonwood, Vermont poplar, Virginia poplar, water poplar, western cottonwood, whitewood, wislizenus cottonwood, yellow cottonwood Populus fremontii-Arizona cottonwood, -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Weeping Willow Salix Babylonica
Weeping willow Salix babylonica Description Introduced to North America as an ornamental. Habit Perennial tree to 40 ft tall; rounded crown; long, hanging branches; grayish-brown, irregularly furrowed bark. Leaves Alternate, simple, very narrowly lance-shaped, finely serrated margin, yellow-green above and milky green below, 3-6 inches in length, 3/8 to 1/2 inch in width. Stems Very slender; smooth; olive-green to pale yellowish brown; hanging or drooping for long distances; almost rope-like; buds are small, appressed and covered by a single, cap-like Source: MISIN. 2021. Midwest Invasive Species Information Network. Michigan State University - Applied Spatial Ecology and Technical Services Laboratory. Available online at https://www.misin.msu.edu/facts/detail.php?id=148. scale; terminal buds lacking. Flowers Dioecious, males and females appear as upright catkins and are quite fuzzy, 1 inch long, appearing before or with the leaves. Fruits and Seeds A 1 in long cluster of valve-like capsules, light brown in color, contains many fine, cottony seeds; ripen in late May to early June. Habitat Native to Asia. Reproduction By herbaceous stem cuttings, woody stem cuttings or softwood cuttings. Similar White Willow (Salix alba); Black Willow (Salix nigra); Corkscrew Willow (Salix matsudana Koidzumi ). Monitoring and Rapid Response Hand pull small seedlings; use machinery to remove larger trees and root systems in dry areas; effectively controlled using any of several readily available general use herbicides such as glyphosate. Credits The information provided in this factsheet was gathered from the USDA PLANTS Database and Virginia Tech Department of Forest Resources and Environmental Conservation VTree. -

Poplars and Willows: Trees for Society and the Environment / Edited by J.G
Poplars and Willows Trees for Society and the Environment This volume is respectfully dedicated to the memory of Victor Steenackers. Vic, as he was known to his friends, was born in Weelde, Belgium, in 1928. His life was devoted to his family – his wife, Joanna, his 9 children and his 23 grandchildren. His career was devoted to the study and improve- ment of poplars, particularly through poplar breeding. As Director of the Poplar Research Institute at Geraardsbergen, Belgium, he pursued a lifelong scientific interest in poplars and encouraged others to share his passion. As a member of the Executive Committee of the International Poplar Commission for many years, and as its Chair from 1988 to 2000, he was a much-loved mentor and powerful advocate, spreading scientific knowledge of poplars and willows worldwide throughout the many member countries of the IPC. This book is in many ways part of the legacy of Vic Steenackers, many of its contributing authors having learned from his guidance and dedication. Vic Steenackers passed away at Aalst, Belgium, in August 2010, but his work is carried on by others, including mem- bers of his family. Poplars and Willows Trees for Society and the Environment Edited by J.G. Isebrands Environmental Forestry Consultants LLC, New London, Wisconsin, USA and J. Richardson Poplar Council of Canada, Ottawa, Ontario, Canada Published by The Food and Agriculture Organization of the United Nations and CABI CABI is a trading name of CAB International CABI CABI Nosworthy Way 38 Chauncey Street Wallingford Suite 1002 Oxfordshire OX10 8DE Boston, MA 02111 UK USA Tel: +44 (0)1491 832111 Tel: +1 800 552 3083 (toll free) Fax: +44 (0)1491 833508 Tel: +1 (0)617 395 4051 E-mail: [email protected] E-mail: [email protected] Website: www.cabi.org © FAO, 2014 FAO encourages the use, reproduction and dissemination of material in this information product. -

"A Most Dangerous Tree": the Lombardy Poplar in Landscape Gardening
"A Most Dangerous Tree": The Lombardy Poplar in Landscape Gardening Christina D. Wood ~ The history of the Lombardy poplar in America illustrates that there are fashions in trees just as in all else. "The Lombardy poplar," wrote Andrew Jackson may have originated in Persia or perhaps the Downing in 1841, "is too well known among Himalayan region; because the plant was not us to need any description."’ This was an ex- mentioned in Roman agricultural texts, writ- traordinary thing to say about a tree that had ers reasoned that it must have been introduced been introduced to North America less than to Italy from central Asia.3 But subsequent sixty years earlier. In that short time, this writers have thought it more likely that the distinctive cultivar of dominating height Lombardy sprang up as a mutant of the black had gained notoriety due to aggressive over- poplar. Augustine Henry found evidence that planting in the years just after its introduction. it originated between 1700 and 1720 in Lom- The Lombardy poplar (Populus nigra bardy and spread worldwide by cuttings, reach- ’Italica’) is a very tall, rapidly growing tree ing France in 1749, England in 1758, and North with a distinctively columnar shape, often America in 1784.4 It was soon widely planted with a buttressed base. It is a fastigiate muta- in Europe as an avenue tree, as an ornamental, tion of a male black poplar (P. nigra).2 As a and for a time, for its timber. According to at member of the willow family (Salicaceae)- least one source, it was used in Italy to make North American members of the genus in- crates for grapes until the early nineteenth clude the Eastern poplar (Populus deltoides), century, when its wood was abandoned for this bigtooth aspen (Populus granditata), and purpose in favor of that of P. -

Poplar Chap 1.Indd
Populus: A Premier Pioneer System for Plant Genomics 1 1 Populus: A Premier Pioneer System for Plant Genomics Stephen P. DiFazio,1,a,* Gancho T. Slavov 1,b and Chandrashekhar P. Joshi 2 ABSTRACT The genus Populus has emerged as one of the premier systems for studying multiple aspects of tree biology, combining diverse ecological characteristics, a suite of hybridization complexes in natural systems, an extensive toolbox of genetic and genomic tools, and biological characteristics that facilitate experimental manipulation. Here we review some of the salient biological characteristics that have made this genus such a popular object of study. We begin with the taxonomic status of Populus, which is now a subject of ongoing debate, though it is becoming increasingly clear that molecular phylogenies are accumulating. We also cover some of the life history traits that characterize the genus, including the pioneer habit, long-distance pollen and seed dispersal, and extensive vegetative propagation. In keeping with the focus of this book, we highlight the genetic diversity of the genus, including patterns of differentiation among populations, inbreeding, nucleotide diversity, and linkage disequilibrium for species from the major commercially- important sections of the genus. We conclude with an overview of the extent and rapid spread of global Populus culture, which is a testimony to the growing economic importance of this fascinating genus. Keywords: Populus, SNP, population structure, linkage disequilibrium, taxonomy, hybridization 1Department of Biology, West Virginia University, Morgantown, West Virginia 26506-6057, USA; ae-mail: [email protected] be-mail: [email protected] 2 School of Forest Resources and Environmental Science, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, USA; e-mail: [email protected] *Corresponding author 2 Genetics, Genomics and Breeding of Poplar 1.1 Introduction The genus Populus is full of contrasts and surprises, which combine to make it one of the most interesting and widely-studied model organisms. -

Populus Nigra L., in Europe
Original article Strategies for the conservation of a pioneer tree species, Populus nigra L., in Europe François Lefèvre* Agnès Légionnet Sven de Vries Jozef Turok a Unite de recherches forestières méditerranéennes, Institut national de la recherche agronomique, 84000 Avignon, France b Station d’amelioration des arbres forestiers, Institut national de la recherche agronomique, 45160 Ardon, France C IBN/DLO, P.O. Box 23, 6700 AA Wageningen, The Netherlands d IPGRI, via delle Sette Chiese 142, 00145 Rome, Italy Abstract - The European black poplar is a pioneer tree species of the riparian ecosys- tem. Its natural habitat is exposed to anthropogenic alteration. Overexploitation of the trees, and interaction with a narrow-based cultivated gene pool also contribute to the decline of the species. National programmes for the conservation of Populus nigra exist in most European countries, and it was elected as one of the pilot species in the EUFORGEN programme. The strategies developed in 17 European countries over the species range, and 3 years of collaborative efforts within the EUFORGEN P. nigra Network are reviewed here. The conservation strategies need to be adapted to the biological characteristics and ecological requirements of black poplar. Applied conser- vation includes ex situ methods for the conservation of genotypes, and, for long-term gene conservation, in situ management of sites in relation to the preexisting natural reserves, or dynamic conservation in the framework of poplar breeding programmes. The social and cultural impact of poplars also interfere with applied conservation. © Inra/Elsevier, Paris Populus / genetic resources / conservation strategy Résumé - Conservation d’une espèce forestière pionnière, Populus nigra I., En Europe. -
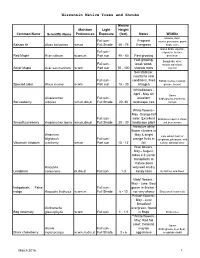
Wisconsin Native Trees and Shrubs
Wisconsin Native Trees and Shrubs Mature Moisture Light Height Common Name Scientific Name Preferences Exposure (feet) Notes Wildlife Grouse, deer, Full sun - Fragrant moose,porcupine, game Balsam fir Abies balsamea wm,m Full Shade 40 - 75 Evergreen birds, mice Game birds, squirrel, Full sun - chipmunk, beaver, Red Maple Acer rubrum w,wm,m Part sun 40 - 60 Fast growing deer,bear Fast growing, Songbirds, deer, Full sun - weak wood, racoon,waterfowl, Silver Maple Acer saccharinum w,wm Part sun 75 - 100 shallow roots squirrel Soil stablizer, neutral to acid Full sun - conditions, fixes Rabbit,moose,muskrat, Specled alder Alnus incana w,wm Part sun 15 - 30 nitrogen grouse, beaver Whiteflowers - April - May An Game Amelanchier Full sun - excellent birds,grouse,skunk,fox, Serviceberry arborea wm,m,dm,d Full Shade 20 -30 landscape tree racoon White flowers - May Orange fall Full sun - color Excellent Birds,bear,squirrel,chipm Smooth juneberry Amelanchier laevis wm,m,dm,d Full Shade 20 - 30 landscape plant unk,deer,moose Attractive white flower clusters in American May & bright Late winter food for Highbush Full sun - orange fruits in songbirds, pheasant, wild Viburnum trilobum cranberry wm,m Part sun 10 - 13' fall turkey, whitetail deer Blue flowers, May - August; takes 2-3 yrs for transplants to mature;does Amorpha very well on dry Leadplant canescens m,dm,d Full sun 1-3 sandy sites Butterflies and Bees Violet flowers - May - June Best Indigobush; False Full sun - grown in thicket - indigo Amorpha fruticosa w,wm,m Full Shade 6 - 12 not very