Recovery of a Wild Rice Stand Following Mechanical Removal of Narrowleaf Cattail
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Form 104 School Lunch Meal Pattern Grains Fact Sheet 6.2015.Pub
School Lunch Meal Pattern Grains Fact Sheet Form 104 All grains must be Whole Grain-Rich (WGR) June 2015 Whole GrainGrain----RichRich (WGR) Barley Wheat (Red) Dehulled barley Bulgur (cracked wheat) DehulledDehulled----barleybarley flour Bromated wholewhole----wheatwheat flour Whole barley Cracked wheat or crushed wheat WholeWhole----barleybarley flakes Entire wheat flour WholeWhole----barleybarley flour Graham flour Whole grain barley Sprouted wheat WholeWhole----graingrain barley flour Sprouted wheat berries Stone ground wholewhole----wheatwheat flour Brown Rice Toasted crushed whole wheat Brown rice Wheat berries Brown rice flour Whole bulgur Whole durum flour Corn Whole durum wheat flour Whole corn WholeWhole----graingrain bulgur WholeWhole----corncorn flour WholeWhole----graingrain wheat Whole cornmeal WholeWhole----wheatwheat flour WholeWhole----graingrain corn flour WholeWhole----wheatwheat pastry flour WholeWhole----graingrain grits Whole wheat flakes Oats Wheat(White) Oat groats Whole white wheat Oatmeal or rolled oats Whole white wheat flour Whole oats WholeWhole----oatoat flour Wild Rice Rye Wild rice Whole rye WildWild----ricerice flour Rye berries WholeWhole----ryerye flour WholeWhole----ryerye flakes Less Common Grains To be whole grains “whole” must be listed before the grain name Amaranth Buckwheat Einkorn Emmer (faro) Kamut ® Millet Quinoa Sorghum (milo) Spelt Teff Triticale Grain Facts: To be considered WGR, the product must contain 100% whole grain OR be at least 50 Form 104 percent whole grains, any remaining grains must be enriched and any non-creditable June 2015 grains must be less than 2 percent (¼ ounce equivalent) of the product formula. For more information, see Whole Grain Resource for NSLP and SBP Manual No more than two grain-based desserts can be credited per week. -

Whole and Enriched Grains CACFP Reference Sheet
OSPI CNS Child and Adult Care Food Program Reference Sheet Whole and Enriched Grains Whole and enriched grains are a part of identifying Whole Grain-Rich (WGR) items. There are several methods to identify WGR items. Please view the Grain Requirements in the CACFP Reference Sheet for more information. Whole Grains: Amaranth Sprouted einkorn Amaranth flour Sprouted spelt Brown rice Sprouted whole rye Buckwheat Sprouted whole wheat Buckwheat flour Steel cut oats Buckwheat groats Teff Bulgur Teff flour Cracked wheat Triticale Graham flour Triticale flour Instant oatmeal Wheat berries Millet Wheat groats Millet flour Whole durum flour Oat groats Whole einkorn berries Old fashioned oats Whole grain corn Quick cooking oats Whole grain corn flour Quinoa Whole grain einkorn flour Rye groats Whole grain oat flour Sorghum Whole grain spelt flour Sorghum flour Whole grain wheat flakes Spelt berries Whole rye flour Sprouted brown rice Whole wheat flour Sprouted buckwheat Wild rice Whole corn Brans and Germs: Corn bran Rye bran Oat bran Wheat bran Rice bran Wheat germ Enriched Grains: Enriched bromated flour Enriched rice Enriched corn flour Enriched rice flour Enriched durum flour Enriched rye flour Enriched durum wheat Enriched wheat flour flour Enriched white flour OSPI CNS November 2018 OSPI CNS Child and Adult Care Food Program Reference Sheet Disregarded Ingredients – May be ignored (typically presented in small amounts) Corn dextrin Tapioca starch Corn starch Wheat dextrin Modified -

Whole Grain Goodness
Nutrition Know-How Child Health Specialty Clinics Whole Grain Goodness What is a whole grain? A whole grain food has all three parts of the grain, and each part has different vitamins and minerals that work together to provide nutrition. Many people only think of wheat and other bread products when they hear the word “grain,” but there are many foods that contain whole grains. Why do whole grains matter? Whole grains are higher in fiber, iron and vitamins than processed grains like white flour. Fiber can help your child pass bowel movements, and iron and vitamins are very important for a child’s growth and development. People who eat more whole grains are less likely to struggle with their weight and other serious health conditions like colon cancer. How much whole grain does my child need? This depends on your child’s age and size. A good guide is to make at least half of your child’s grain servings whole grain. For example, if your child eats 6 servings of grain, at least 3 of those servings should be whole grain. How do I know if a food is whole grain? Look for the words “whole grain” on the package. Foods with at least 51% whole grain will have a special label on them like the one shown at the top of this page. You can also check the ingredients; to be a whole grain product a food should have a whole grain listed as the first ingredient. A whole grain bread will have “whole wheat flour” as the first ingredient. -

Hybridization Dynamics of Invasive Cattail (Typhaceae) Stands at Pierce Cedar Creek Institute: a Molecular Analysis
HYBRIDIZATION DYNAMICS OF INVASIVE CATTAIL (TYPHACEAE) STANDS AT PIERCE CEDAR CREEK INSTITUTE: A MOLECULAR ANALYSIS Alex Graeff, Kelsey Huisman, and Dr. Pamela J. Laureto Department of Biological Sciences Grand Rapids Community College 143 Bostwick NE Grand Rapids, Michigan 49503 ABSTRACT Three cattail taxa are recognized in Michigan USA: native Typha latifolia (broad-leaf cattail), the invasive Typha angustifolia (narrow-leaf cattail), and the hybrid of these two species Typha × glauca. Typha angustifolia and T. × glauca are of special interest because of their ability to aggressively spread and out-compete the native cattail T. latifolia. Typha × glauca has been shown to out-compete both its parental taxa and produce monospecific stands. We surveyed the Pierce Cedar Creek Institute (PCCI) property for cattails and located 25 distinct cattail marshes. We determined the total area of cattail marsh at PCCI to be roughly 10% of the 267 ha property. Cattail individuals were sampled from each of the 25 stands and random amplified polymorphic DNA markers were used to identify the individuals to species. We found that 20 of the 25 stands were monospecific for the native cattail, T. latifolia. Five of the stands were mixtures of the native T. latifolia and the introduced T. angustifolia, and T. × glauca was found in two of the mixed stands. We recommend removal of the invasive T. angustifolia and T. × glauca individuals and the establishment of a monitoring plan in order to maintain the long-term health of the cattail marshes at PCCI. Keywords: Typha spp., RAPD markers, invasive species 1 INTRODUCTION Species of Typha L. (Typhaceae), commonly known as cattails, are highly productive emergent plants that grow in a variety of wetland habitats throughout the world (McManus et al. -

Water Management of the Steep Rock Iron Mines at Atikokan, Ontario During Construction, Operations, and After Mine Abandonment
Proceedings of the 25th Annual British Columbia Mine Reclamation Symposium in Campbell River, BC, 2001. The Technical and Research Committee on Reclamation WATER MANAGEMENT OF THE STEEP ROCK IRON MINES AT ATIKOKAN, ONTARIO DURING CONSTRUCTION, OPERATIONS, AND AFTER MINE ABANDONMENT V. A. Sowa, P. Eng., F.E.I.C. 1 R. B. Adamson, P. Eng.2 A.W. Chow, P. Eng.3 1 Jacques Whitford and Associates Limited, Vancouver, British Columbia 2 Adamson Consulting, Thunder Bay, Ontario 3 Northwest Region, Ontario Ministry of Natural Resources, Thunder Bay, Ontario ABSTRACT The Steep Rock Iron Mines at Atikokan, Ontario operated from 1944 to 1979. The iron ore was located at the bottom of Steep Rock Lake and water management was a key factor in developing the mines. Open pit mining required a massive water diversion scheme, including the diversion of the Seine River, draining of Steep Rock Lake, and construction of various dams and other diversion structures. In order to abandon the mine, the Province of Ontario required a suitable abandonment and long-term water management plan, and assessment of the condition of the various water control and diversion structures. Reclamation of the Seine River to its original course was not possible and, consequently, the water control structures, primarily dams and tunnels, will be operating in perpetuity. The water management during the development of the mines and during operations is described, as well as some insight into future water management options after abandonment. INTRODUCTION The richest undeveloped deposit of hematite iron ore on the North American continent at the time was discovered in 1938 beneath Steep Rock Lake, near Atikokan, Ontario. -

The Ayurvedic Pharmacopoeia of India
THE AYURVEDIC PHARMACOPOEIA OF INDIA PART- I VOLUME – V GOVERNMENT OF INDIA MINISTRY OF HEALTH AND FAMILY WELFARE DEPARTMENT OF AYUSH Contents | Monographs | Abbreviations | Appendices Legal Notices | General Notices Note: This e-Book contains Computer Database generated Monographs which are reproduced from official publication. The order of contents under the sections of Synonyms, Rasa, Guna, Virya, Vipaka, Karma, Formulations, Therapeutic uses may be shuffled, but the contents are same from the original source. However, in case of doubt, the user is advised to refer the official book. i CONTENTS Legal Notices General Notices MONOGRAPHS Page S.No Plant Name Botanical Name No. (as per book) 1 ËMRA HARIDRË (Rhizome) Curcuma amada Roxb. 1 2 ANISÍNA (Fruit) Pimpinella anisum Linn 3 3 A×KOLAH(Leaf) Alangium salviifolium (Linn.f.) Wang 5 4 ËRAGVËDHA(Stem bark) Cassia fistula Linn 8 5 ËSPHOÙË (Root) Vallaris Solanacea Kuntze 10 6 BASTËNTRÌ(Root) Argyreia nervosa (Burm.f.)Boj. 12 7 BHURJAH (Stem Bark) Betula utilis D.Don 14 8 CAÛÚË (Root) Angelica Archangelica Linn. 16 9 CORAKAH (Root Sock) Angelica glauca Edgw. 18 10 DARBHA (Root) Imperata cylindrica (Linn) Beauv. 21 11 DHANVAYËSAH (Whole Plant) Fagonia cretica Linn. 23 12 DRAVANTÌ(Seed) Jatropha glandulifera Roxb. 26 13 DUGDHIKË (Whole Plant) Euphorbia prostrata W.Ait 28 14 ELAVËLUKAê (Seed) Prunus avium Linn.f. 31 15 GAÛÚÌRA (Root) Coleus forskohlii Briq. 33 16 GAVEDHUKA (Root) Coix lachryma-jobi LInn 35 17 GHOÛÙË (Fruit) Ziziphus xylopyrus Willd. 37 18 GUNDRËH (Rhizome and Fruit) Typha australis -

Gluten-Free Grains
Gluten-Free Grains Amaranth Updated February 2021 Buckwheat The gluten-free diet requires total avoidance of the grains wheat, barley, rye and all varieties and hybrids of these grains, such as spelt. However, there are many wonderful gluten-free grains* to enjoy. Cornmeal, Amaranth Polenta, Grits, Once the sacred food of the Aztecs, amaranth is high in protein, calcium, iron, and fiber. Toasting this tiny grain before cooking brings out its nutty flavor. Hominy Makes a delicious, creamy hot breakfast cereal. Serve with fruit of choice on top and/or a touch of maple syrup. Millet Rice Rice comes in many varieties: short grain, long grain, jasmine and basmati to name a Oats few. Long grain rice tends to be fluffier while short grain rice is stickier. Rice also comes in various colors: black, purple, brown, and red. These colorful un-refined rices contribute more nutritional benefits than does refined white rice and have subtly unique flavors and Quinoa textures too. Wild rice is another different and delicious option. Versatile rice leftovers can go in many directions. Add to salads or sautéed vegetables; Rice make rice pancakes or rice pudding; season and use as filling for baked green peppers or winter squash. Sorghum Buckwheat Despite the name, buckwheat is a gluten-free member of the rhubarb family. Roasted buckwheat is called kasha. Buckwheat is high in B Vitamins, fiber, iron, magnesium, Teff phosphorous and zinc. Buckwheat has an earthy, nutty, slightly bitter taste. Experiment with using the cooked grain (buckwheat “groats”, or “kasha” which is the toasted version) as you would rice. -
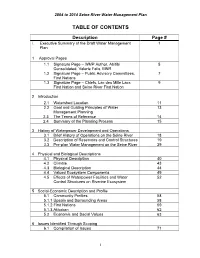
Table of Contents
2004 to 2014 Seine River Water Management Plan _________________________________________________________________ TABLE OF CONTENTS Description Page # i. Executive Summary of the Draft Water Management 1 Plan 1 Approval Pages 1.1 Signature Page – WMP Author, Abitibi 5 Consolidated, Valerie Falls, MNR 1.2 Signature Page – Public Advisory Committees, 7 First Nations 1.3 Signature Page – Chiefs, Lac des Mille Lacs 9 First Nation and Seine River First Nation 2 Introduction 2.1 Watershed Location 11 2.2 Goal and Guiding Principles of Water 13 Management Planning 2.3 The Terms of Reference 14 2.4 Summary of the Planning Process 15 3 History of Waterpower Development and Operations 3.1 Brief History of Operations on the Seine River 18 3.2 Description of Reservoirs and Control Structures 19 3.3 Pre-plan Water Management on the Seine River 29 4 Physical and Biological Descriptions 4.1 Physical Description 40 4.2 Climate 43 4.3 Biological Description 44 4.4 Valued Ecosystem Components 49 4.5 Effects of Waterpower Facilities and Water 52 Control Structures on Riverine Ecosystem 5 Social-Economic Description and Profile 5.1 Community Profiles 58 5.1.1 Upsala and Surrounding Areas 58 5.1.2 First Nations 59 5.1.3 Atikokan 62 5.2 Economic and Social Values 63 6 Issues Identified Through Scoping 6.1 Compilation of Issues 71 i 2004 to 2014 Seine River Water Management Plan _________________________________________________________________ 6.2 Spatial & Temporal Assessment 79 6.3 Issues not addressed in Planning 80 7 Plan Objectives 7.1 Developing the Objectives -

Study of Structure and Properties of Tunisian Typha Leaf Fibers
International Journal of Engineering Research & Technology (IJERT) ISSN: 2278-0181 Vol. 3 Issue 3, March - 2014 Study of Structure and Properties of Tunisian Typha Leaf Fibers Rezig Sana1, Jaouadi Mounir 2 , Msahli Slah3 1 PhD, Laboratory of Textile Engineering, University of Monastir, Tunisia 2 PhD, Laboratory of Textile Engineering, University of Monastir, Tunisia 3 Professor, Laboratory of Textile Engineering, University of Monastir, Tunisia Abstract —Typha fiber is derived from the leaves of Typha some researchers have tried to extract and develop Typha plant, which belongs to the family Typhaceae. The fiber has been fibers of different varieties in the world, the Tunisian Typha obtained using a hot alkali treatment. Some properties of this variety has not been described in the literature. fiber, such as diameter, linear density, tensile properties and thermal properties have been determined. In this study, we attempted to extract the Typha fibers and Using scanning electron microscope, the morphological structure remove non-cellulosic materials using a hot alkali treatment. of the obtained fibers has been investigated and shows a Fiber quality has been evaluated in terms of extraction yield, structure similar to a natural composite of ultimate fiber bundles diameter measurement, linear density, morphological and of cellulose. As a result, measured properties have been mechanical properties. Thermal analysis and Infrared spectrum compared with untreated typha fibers and other lignocellulosic have also been investigated, since fiber performance depends fibers in order to conclude on the importance of the textile potential of the Typha leaf fibers. on its chemical composition and physical properties. As a result, measured properties have been compared with untreated Keywords—Typha fiber; hot alkali treatment; thermal properties; Typha fibers and other lignocellulosic fibers in order to tensile properties; morphological structure. -

Protecting Minnesota's Wild Rice Lakes
Protecting Minnesota’s wild rice lakes June 2015 Snapshots Minnesota is the epicenter of the world’s natural wild rice. Protected, undeveloped shoreland is important to preserving sensitive wild rice lakes for current and future generations of wildlife and outdoor enthusiasts. Although once found throughout most of the state, today, the heart of the state’s wild rice acreage falls within eleven counties: Aitkin, Becker, Beltrami, Carlton, Cass, Clearwater, Crow Wing, Hubbard, Itasca, St. Louis and Wadena. Wild rice is important both socially and culturally in Minnesota. Wild rice also provides important ecological benefits. Wild rice shoreland encompasses a complex of shallow lakes, rivers, and shallow bays of deeper lakes that support rice and provide some of the most important habitat for wetland- dependent wildlife species in Minnesota. Wild rice habitat is especially important to Minnesota’s migrating and breeding waterfowl and provides Minnesotans with unique recreation opportunities: hunting waterfowl and harvesting the rice itself for food. Wild rice also improves and protects water A young man harvests wild quality by keeping soil and nutrients in place and acting as a buffer to slow rice. winds across wetlands. The Minnesota Board of Water and Soil Resources (BWSR) has received Outdoor Heritage Funds to support and protect our state grain. Working in cooperation with the DNR and soil and water conservation districts, BWSR will complete 46 easement projects on 29 lakes and rivers. Funding for wild rice protection began in 2012. This first phase of the project was awarded $1.89 million which yielded 18 completed projects extending permanent protection to almost 10 miles of wild rice shoreland. -

Potential Benefits and Toxicity of Nanoselenium and Nitric Oxide in Peppermint
doi:10.14720/aas.2018.111.2.11 Original research article / izvirni znanstveni članek Potential benefits and toxicity of nanoselenium and nitric oxide in peppermint Hossein NAZERIEH1, Zahra Oraghi ARDEBILI1*, Alireza IRANBAKHSH2 Received January 13, 2018; accepted May 04, 2018. Delo je prispelo 13. januarja 2018, sprejeto 04. maja 2018. ABSTRACT IZVLEČEK Taking account of nano-compounds and biofortification, this POTENCIALNE KORISTI IN STRUPENOST research was conducted to evaluate peppermint (Mentha x NANOSELENA IN DUŠIKOVEGA OKSIDA PRI piperita L.) responses to nano-selenium (nSe; 0, 2, and POPROVI METI 20 mg l-1) and/or nitric oxide (NO; 0 and 8 mg l-1). Significant increases in leaf length, and area, and shoot fresh mass were Raziskava je bila izvedena za ovrednostenje odziva poprove enhanced by the low level of nSe and/or NO, contrasted with mete (Mentha x piperita L.) na nano selen (nSe; 0, 2, in the high dose. The inhibitory effects of the high dose of nSe 20 mg l-1) in/ali dušikov oksid (NO; 0 in 8 mg l-1). Značilno on the growth-related characteristics were significantly povečanje dolžine in površine listov in sveže mase poganjkov mitigated by NO. The adverse impact of nSe20 on chlorophyll je bilo vzpodbujeno z majhnimi količinami nSe in/ali NO, concentration was alleviated by NO. The individual and nasprotno od učinkov velikih količin. Zaviralni učinki velikih combined treatments of nSe2 led to the significant inductions koncentracij nSe na z rastjo povezane parametre so bili in the activities of nitrate reductase and peroxidase, whereas značilno zmanjšani z dodatkom NO. -

Expanded Understanding of Eleusine Diversity and Evolution
Expanded Understanding of Eleusine Diversity and Evolution by Hui Zhang A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama August 4, 2018 Key words: Next-generation sequencing, Illumina, Genome, Transcriptome, Phylogenetic, Goosegrass Copyright 2018 by Hui Zhang Approved by J. Scott McElroy, Chair, Professor of Crop, Soil and Environmental Sciences Charles Y. Chen, Professor of Crop, Soil and Environmental Sciences Leslie R. Goertzen, Associate Professor of Biological Sciences Eric Peatman, Associate Professor of Fisheries, Aquaculture, and Aquatic Sciences i Abstract Eleusine, including 9 to 12 species, is a small genus of annual and perennial grass species within the Eragrosteae tribe and Chloridoideae subfamily. There are very few genomic information about this genus. The primary goal of this dissertation research is to expand understanding of Eleusine diversity and evolution. The dissertation opens with a brief literature review regarding the motivation for this research. In chapter 2 we report a draft assembly of approximately 498 Mb whole-genome sequences of goosegrass obtained by de novo assembly of paired-end and mate-paired reads generated by Illumina sequencing of total genomic DNA. From around 88 GB of the clean data, the genome was assembled into 24,063 scaffolds with N50 = 233,823 bp. The nuclear genome assembly contains 25,467 predicted unique protein-coding genes. Sixteen target herbicide resistant genes and four non-target herbicide resistant gene families were obtained from this draft genome. Chapter 3 presents a complete plastid genome sequence of goosegrass obtained by de novo assembly of paired-end and mate-paired reads obtained in chapter 2.