Sleep and Nesting Behavior in Primates: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predator-Primate Occupation and Co-Occurrence in the Issa Valley, Katavi Region, Western Tanzania
BSc. thesis: Predator-primate occupation and co-occurrence in the Issa Valley, Katavi Region, western Tanzania. Menno J. Breider Student Applied Biology, Aeres University of Applied Sciences, Almere, The Netherlands Aeres University graduation teacher: Quirine Hakkaart In association with the Ugalla Primate Project Edam, 2 June 2017 This page is intentionally left blank for double-sided printing BSc. thesis: Predator-primate occupation and co-occurrence in the Issa Valley, Katavi Region, western Tanzania. Menno J. Breider Student Applied Biology, Aeres University of Applied Sciences, Almere, The Netherlands Aeres University graduation teacher: Quirine Hakkaart In association with the Ugalla Primate Project Edam, 2 June 2017 Front page images, top to bottom: Top: Eastern chimpanzee and leopard at the same location, different occasions. Middle: Researcher and leopard at the same location, different occasions. Bottom: Red-tailed monkey and researchers at the same location, different occasions. All: Camera trap footage from the Issa Valley, provided by the Ugalla Primate Project. Edited: combined, gradient created and cropped. This page is intentionally left blank for double-sided printing - Acknowledgements I wish to thank, first and foremost, Alex Piel of the Ugalla Primate Project for enabling this subject and for patiently supporting me during this project. His quick responses (often within an hour, no matter what time of the day), feedback and insights have been indispensable. I am also grateful to my graduation teacher, Quirine Hakkaart from the Aeres University, for guiding me through this thesis project and for her feedback on multiple versions of this study and its proposal. I would also have been unable to complete this research without the support and feedback of my friends and family. -

Sexual Selection in the Ring-Tailed Lemur (Lemur Catta): Female
Copyright by Joyce Ann Parga 2006 The Dissertation Committee for Joyce Ann Parga certifies that this is the approved version of the following dissertation: Sexual Selection in the Ring-tailed Lemur (Lemur catta): Female Choice, Male Mating Strategies, and Male Mating Success in a Female Dominant Primate Committee: Deborah J. Overdorff, Supervisor Claud A. Bramblett Lisa Gould Rebecca J. Lewis Liza J. Shapiro Sexual Selection in the Ring-tailed Lemur (Lemur catta): Female Choice, Male Mating Strategies, and Male Mating Success in a Female Dominant Primate by Joyce Ann Parga, B.S.; M.A. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin December, 2006 Dedication To my grandfather, Santiago Parga Acknowledgements I would first like to thank the St. Catherines Island Foundation, without whose cooperation this research would not have been possible. The SCI Foundation graciously provided housing and transportation throughout the course of this research. Their aid is gratefully acknowledged, as is the permission to conduct research on the island that I was granted by the late President Frank Larkin and the Foundation board. The Larkin and Smith families are also gratefully acknowledged. SCI Superintendent Royce Hayes is thanked for his facilitation of all aspects of the field research, for his hospitality, friendliness, and overall accessibility - whether I was on or off the island. The Wildlife Conservation Society (WCS) is also an organization whose help is gratefully acknowledged. In particular, Colleen McCann and Jim Doherty of the WCS deserve thanks. -

001 Emma the Bunny #006 Georgina the Hippo #011 Germaine
#001 #002 #003 #004 #005 Emma the bunny Alexandre the russian Piotr the polar bear Bridget the elephant Simon the sheep blue cat #006 #007 #008 #009 #010 Georgina the hippo Seamus the alpaca Austin the rhino Rufus the lion Richard the large white pig #011 #012 #013 #014 #015 Germaine the gorilla Winston the aardvark Penelope the bear Hank the dorset Fiona the panda down sheep #016 #017 #018 #019 #020 Juno the siamese cat Angharad the donkey Benedict the chimpanzee Samuel the koala Douglas the highland cow #021 #022 #023 #024 #025 Laurence the tiger Chardonnay the Claudia the Alice the zebra Audrey the nanny goat palomino pony saddleback pig #026 #027 #028 #029 #030 Clarence the bat Martin the tabby cat Sarah the friesian cow Timmy the jack russell Caitlin the giraffe #031 #032 #033 #034 #035 Esme the fox Blake the orangutan Siegfried the monkey Boris the red squirrel Hamlet the cheetah #036 #037 #038 #039 #040 Francis the hedgehog Jessie the raccoon Bradlee the grey squirrel Noah the zwartbles sheep Christophe the wolf #041 #042 #043 #044 #045 Sheila the kangaroo Andre the lemur Erica the Frank the armadillo Mae the snow leopard dromedary camel #046 #047 #048 #049 #050 Harriet the sloth Susan the badger Zack the skunk Donna the reindeer Logan the moose #051 #052 #053 #054 #055 Simone the suri alpaca Fred the herdwick sheep Eustice the beltex sheep Tobias the Seth the hebridean sheep wensleydale sheep #056 #057 #058 #059 #060 Tracy the racka sheep Xavier the gibbon Sid the giant anteater Caroline the platypus Harold the teeswater sheep -

Black Internationalism and African and Caribbean
BLACK INTERNATIONALISM AND AFRICAN AND CARIBBEAN INTELLECTUALS IN LONDON, 1919-1950 By MARC MATERA A Dissertation submitted to the Graduate School-New Brunswick Rutgers, the State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in History Written under the direction of Professor Bonnie G. Smith And approved by _______________________ _______________________ _______________________ _______________________ New Brunswick, New Jersey May 2008 ABSTRACT OF THE DISSERTATION Black Internationalism and African and Caribbean Intellectuals in London, 1919-1950 By MARC MATERA Dissertation Director: Bonnie G. Smith During the three decades between the end of World War I and 1950, African and West Indian scholars, professionals, university students, artists, and political activists in London forged new conceptions of community, reshaped public debates about the nature and goals of British colonialism, and prepared the way for a revolutionary and self-consciously modern African culture. Black intellectuals formed organizations that became homes away from home and centers of cultural mixture and intellectual debate, and launched publications that served as new means of voicing social commentary and political dissent. These black associations developed within an atmosphere characterized by a variety of internationalisms, including pan-ethnic movements, feminism, communism, and the socialist internationalism ascendant within the British Left after World War I. The intellectual and political context of London and the types of sociability that these groups fostered gave rise to a range of black internationalist activity and new regional imaginaries in the form of a West Indian Federation and a United West Africa that shaped the goals of anticolonialism before 1950. -

The Biogeography of Large Islands, Or How Does the Size of the Ecological Theater Affect the Evolutionary Play
The biogeography of large islands, or how does the size of the ecological theater affect the evolutionary play Egbert Giles Leigh, Annette Hladik, Claude Marcel Hladik, Alison Jolly To cite this version: Egbert Giles Leigh, Annette Hladik, Claude Marcel Hladik, Alison Jolly. The biogeography of large islands, or how does the size of the ecological theater affect the evolutionary play. Revue d’Ecologie, Terre et Vie, Société nationale de protection de la nature, 2007, 62, pp.105-168. hal-00283373 HAL Id: hal-00283373 https://hal.archives-ouvertes.fr/hal-00283373 Submitted on 14 Dec 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THE BIOGEOGRAPHY OF LARGE ISLANDS, OR HOW DOES THE SIZE OF THE ECOLOGICAL THEATER AFFECT THE EVOLUTIONARY PLAY? Egbert Giles LEIGH, Jr.1, Annette HLADIK2, Claude Marcel HLADIK2 & Alison JOLLY3 RÉSUMÉ. — La biogéographie des grandes îles, ou comment la taille de la scène écologique infl uence- t-elle le jeu de l’évolution ? — Nous présentons une approche comparative des particularités de l’évolution dans des milieux insulaires de différentes surfaces, allant de la taille de l’île de La Réunion à celle de l’Amé- rique du Sud au Pliocène. -

ANNEX 3 ICC-01/09-02/11-67-Anx3 21-04-2011 2/84 EO PT
ICC-01/09-02/11-67-Anx3 21-04-2011 1/84 EO PT No. ICC-01/09-02/11 21-4-11 ANNEX 3 ICC-01/09-02/11-67-Anx3 21-04-2011 2/84 EO PT A PROGRESS REPORT TO THE HON. ATTORNEY-GENERAL BY THE TEAM ON UPDATE OF POST ELECTION VIOLENCE RELATED CASES IN WESTERN, NYANZA, CENTRAL, RIFT-VALLEY, EASTERN, COAST AND NAIROBI PROVINCES MARCH, 2011 NAIROBI ICC-01/09-02/11-67-Anx3 21-04-2011 3/84 EO PT TABLE OF CONTENTS CHAPTER SUBJECT PAGE TRANSMITTAL LETTER IV 1. INTRODUCTION 1 2. GENDER BASED VIOLENCE CASES 7 3. WESTERN PROVINCE 24 3. RIFT VALLEY PROVINCE 30 4. NYANZA PROVINCE 47 5. COAST PROVINCE 62 6. NAIROBI PROVINCE 66 7. CENTRAL PROVINCE 69 8. STATISTICAL ANALYSIS 70 9. CONCLUSION 73 10. APPENDICES ICC-01/09-02/11-67-Anx3 21-04-2011 4/84 EO PT APPENDIX (NO.) LIST OF APPENDICES APP. 1A - Memo from CPP to Hon. Attorney General APP.1B - Memo from CPP to Hon. Attorney General APP.1C - Update on 2007 Post Election Violence offences As at 4th March, 2010 (police commissioner’s report) APP. 1D - Update by Taskforce on Gender Based Violence Cases (police commissioner’s report) APP. 2 - Memo to Solicitor- General from CPP APP. 3 - Letter from PCIO Western APP. 4 - Letter from PCIO Rift Valley APP.5 - Cases Pending Under Investigations in Rift Valley on special interest cases APP.6 - Cases where suspects are known in Rift Valley but have not been arrested APP.7 - Letter from PCIO Nyanza APP.8 - Letter from PCIO Coast APP.9 - Letter from PCIO Nairobi APP.10 - Correspondences from the team ICC-01/09-02/11-67-Anx3 21-04-2011 5/84 EO PT The Hon. -

High School Band Required List
2021-2022 High School Band Required List Table of Contents Click on the links below to jump to that list Band Group I (Required) Group II (Required) Group III (Required) BAND 2021‐2022 ISSMA REQUIRED LIST HIGH SCHOOL Perform as published unless otherwise specified. OOP = Permanently Out of Print * = New Additions or Changes this year Approximate times indicated for most selections GROUP I (REQUIRED) Aegean Festival Overture Makris / Bader E.C. Schirmer 10:00 Aerial Fantasy Michael A. Mogensen Barnhouse 10:30 Al Fresco Karel Husa Associated Music 12:13 American Faces David R. Holsinger TRN 6:00 American Hymnsong Suite (mvts. 1 & 3 or mvts. 1 & 4) Dwayne Milburn Kjos 3:11 / 1:45 / 1:50 American Overture For Band Joseph Willcox Jenkins Presser 115‐40040 5:00 American Salute Gould / Lang Belwin / Alfred 4:45 And The Mountains Rising Nowhere Joseph Schwantner European American Music 12:00 And The Multitude With One Voice Spoke James Hosay Curnow 8:00 Angels In The Architecture Frank Ticheli Manhattan Beach 15:00 Apotheosis Kathryn Salfelder Kon Brio 5:45 Apotheosis Of This Earth Karel Husa Associated Music 8:38 Archangel Raphael Leaves A House Of Tobias Masanori Taruya Foster Music 9:00 Armenian Dances Part I Alfred Reed Sam Fox / Plymouth 10:30 Armenian Dances Part II Alfred Reed Barnhouse 9:30 Army Of The Nile (March) Alford or Alford / Fennell Boosey & Hawkes 3:00 Aspen Jubilee Ron Nelson Boosey & Hawkes 10:00 Asphalt Cocktail John Mackey Osti Music 6:00 Austrian Overture Thomas Doss DeHaske 10:20 Away Day A. -

The Legal Apprentice
sja1-1_cv_sja1-1_cv 3/31/2014 2:40 PM Page 2 THE LEGAL APPRENTICE FOREWORD Dean Kathleen Voute’ MacDonald GOOD LEGAL WRITING IS SIMPLY GOOD WRITING: WORDS OF WELCOME Chief Judge Loretta A. Preska ARTICLES CONSTITUTIONAL BANS ON ALCOHOL ADS IN COLLEGE PRESS Shada Paula STARBUCKS’ BARRISTAS’ BATTLE: TIP POOL SHARING James Baek THE CASE OF THE CONNECTICUT CHIMP: STRICT LIABILITY FOR DOMESTICALLY KEPT WILD ANIMALS Jessica Fusco PROFESSIONAL PHOTOGRAPHER’S LIABILITY FOR REFUSAL TO WORK A SAME-SEX WEDDING Arturo Pena A LEGAL WRITING RESOURCE: THE BIBLE Professor Mary Noe Volume 1 Spring 2014 Number 1 sja1-1_cv_sja1-1_cv 3/31/2014 2:40 PM Page 3 The views expressed in the articles are to be attributed solely to their authors and not to St. John’s University or College of Professional Studies. Submissions to The Legal Apprentice or for additional copies contact [email protected]. ST. JOHN’S UNIVERSITY COLLEGE OF PROFESSIONAL STUDIES KATHLEEN VOUTE´ MACDONALD, ED.D. DEAN ELLEN TUFANO, PH.D. ASSOCIATE DEAN * * * JEFFREY GROSSMAN, J.D. CHAIRPERSON CRIMINAL JUSTICE, LEGAL STUDIES, HOMELAND SECURITY BERNARD HELLDORFER, J.D. DIRECTOR LEGAL STUDIES MARY NOE, J.D. ASSOCIATE PROFESSOR EDITOR JAMES CROFT, J.D. ASSISTANT PROFESSOR ASSISTANT EDITOR THE LEGAL APPRENTICE FACULTY ADVISORY BOARD JAMES CROFT, ASSISTANT PROFESSOR B.A., J. D. ALBANY UNIVERSITY ST. JOHN’S UNIVERSITY, SCHOOL OF LAW JUDITH CRAMER, ASSOCIATE PROFESSOR B.S., M.A., PH.D. UNIVERSITY OF HARTFORD THE UNION INSTITUTE AND UNIVERSITY, CINCINNATI, OH. SUSAN LUSHING, ASSOCIATE PROFESSOR B.S., J.D. CORNELL UNIVERSITY NEW YORK UNIVERSITY, SCHOOL OF LAW MARY NOE, ASSOCIATE PROFESSOR B.A., J.D. -
PROVISIONAL LIST.Pdf
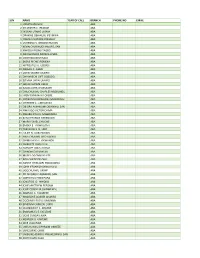
S/N NAME YEAR OF CALL BRANCH PHONE NO EMAIL 1 JONATHAN FELIX ABA 2 SYLVESTER C. IFEAKOR ABA 3 NSIKAK UTANG IJIOMA ABA 4 ORAKWE OBIANUJU IFEYINWA ABA 5 OGUNJI CHIDOZIE KINGSLEY ABA 6 UCHENNA V. OBODOCHUKWU ABA 7 KEVIN CHUKWUDI NWUFO, SAN ABA 8 NWOGU IFIONU TAGBO ABA 9 ANIAWONWA NJIDEKA LINDA ABA 10 UKOH NDUDIM ISAAC ABA 11 EKENE RICHIE IREMEKA ABA 12 HIPPOLITUS U. UDENSI ABA 13 ABIGAIL C. AGBAI ABA 14 UKPAI OKORIE UKAIRO ABA 15 ONYINYECHI GIFT OGBODO ABA 16 EZINMA UKPAI UKAIRO ABA 17 GRACE UZOME UKEJE ABA 18 AJUGA JOHN ONWUKWE ABA 19 ONUCHUKWU CHARLES NSOBUNDU ABA 20 IREM ENYINNAYA OKERE ABA 21 ONYEKACHI OKWUOSA MUKOSOLU ABA 22 CHINYERE C. UMEOJIAKA ABA 23 OBIORA AKINWUMI OBIANWU, SAN ABA 24 NWAUGO VICTOR CHIMA ABA 25 NWABUIKWU K. MGBEMENA ABA 26 KANU FRANCIS ONYEBUCHI ABA 27 MARK ISRAEL CHIJIOKE ABA 28 EMEKA E. AGWULONU ABA 29 TREASURE E. N. UDO ABA 30 JULIET N. UDECHUKWU ABA 31 AWA CHUKWU IKECHUKWU ABA 32 CHIMUANYA V. OKWANDU ABA 33 CHIBUEZE OWUALAH ABA 34 AMANZE LINUS ALOMA ABA 35 CHINONSO ONONUJU ABA 36 MABEL OGONNAYA EZE ABA 37 BOB CHIEDOZIE OGU ABA 38 DANDY CHIMAOBI NWOKONNA ABA 39 JOHN IFEANYICHUKWU KALU ABA 40 UGOCHUKWU UKIWE ABA 41 FELIX EGBULE AGBARIRI, SAN ABA 42 OMENIHU CHINWEUBA ABA 43 IGNATIUS O. NWOKO ABA 44 ICHIE MATTHEW EKEOMA ABA 45 ICHIE CORDELIA CHINWENDU ABA 46 NNAMDI G. NWABEKE ABA 47 NNAOCHIE ADAOBI ANANSO ABA 48 OGOJIAKU RUFUS UMUNNA ABA 49 EPHRAIM CHINEDU DURU ABA 50 UGONWANYI S. AHAIWE ABA 51 EMMANUEL E. -

Pre-Sleep and Sleeping Platform Construction Behavior in Captive Orangutans (Pongo Spp
Original Article Folia Primatol 2015;86:187–202 Received: July 28, 2014 DOI: 10.1159/000381056 Accepted after revision: February 18, 2015 Published online: May 13, 2015 Pre-Sleep and Sleeping Platform Construction Behavior in Captive Orangutans (Pongo spp. ): Implications for Ape Health and Welfare a b–d David R. Samson Rob Shumaker a b Department of Evolutionary Anthropology, Duke University, Durham, N.C., Indianapolis c Zoo, VP of Conservation and Life Sciences, Indianapolis, Ind. , Indiana University, d Bloomington, Ind. , and Krasnow Institute at George Mason University, Fairfax, Va. , USA Key Words Orangutan · Sleep · Sleeping platform · Nest · Animal welfare · Ethology Abstract The nightly construction of a ‘nest’ or sleeping platform is a behavior that has been observed in every wild great ape population studied, yet in captivity, few analyses have been performed on sleep related behavior. Here, we report on such behavior in three female and two male captive orangutans (Pongo spp.), in a natural light setting, at the Indianapolis Zoo. Behavioral samples were generated, using infrared cameras for a total of 47 nights (136.25 h), in summer (n = 25) and winter (n = 22) periods. To characterize sleep behaviors, we used all-occurrence sampling to generate platform construction episodes (n = 217). Orangutans used a total of 2.4 (SD = 1.2) techniques and 7.5 (SD = 6.3) actions to construct a sleeping platform; they spent 10.1 min (SD – 9.9 min) making the platform and showed a 77% preference for ground (vs. elevated) sleep sites. Com- parisons between summer and winter platform construction showed winter start times (17:12 h) to be significantly earlier and longer in duration than summer start times (17:56 h). -

Logical Inferences from Visual and Auditory Information in Ruffed Lemurs and Sifakas
Animal Behaviour xxx (xxxx) xxx Contents lists available at ScienceDirect Animal Behaviour journal homepage: www.elsevier.com/locate/anbehav Logical inferences from visual and auditory information in ruffed lemurs and sifakas * Francesca De Petrillo a, b, , Alexandra G. Rosati b, c a Institute for Advance Study in Toulouse, 1, Esplanade de l'Universite, Toulouse, France b Department of Psychology, University of Michigan, Ann Arbor, MI, U.S.A. c Department of Anthropology, University of Michigan, Ann Arbor, MI, U.S.A. article info Inference by exclusion, or the ability to select a correct course of action by systematically excluding other Article history: potential alternatives, is a form of logical inference that allows individuals to solve problems without Received 17 October 2019 complete information. Current comparative research shows that several bird, mammal and primate Initial acceptance 24 December 2019 species can find hidden food through inference by exclusion. Yet there is also wide variation in how Final acceptance 31 January 2020 successful different species are as well as the kinds of sensory information they can use to do so. An Available online xxx important question is therefore why some species are better at engaging in logical inference than others. MS. number: A19-00797R Here, we investigate the evolution of logical reasoning abilities by comparing strepsirrhine primate species that vary in dietary ecology: frugivorous ruffed lemurs (Varecia spp.) and folivorous Coquerel's Keywords: sifakas, Propithecus coquereli. Across two studies, we examined their abilities to locate food using direct auditory information information versus inference from exclusion and using both visual and auditory information. -

Pen International Writers in Prison Committee Caselist
PEN INTERNATIONAL WRITERS IN PRISON COMMITTEE CASELIST January-December 2013 PEN International Writers in Prison Committee 50/51 High Holborn London WC1V 6ER United Kingdom Tel: + 44 020 74050338 Fax: + 44 020 74050339 e-mail: [email protected] web site: www.pen-international.org PEN INTERNATIONAL CHARTER The P.E.N. Charter is based on resolutions passed at its International Congresses and may be summarised as follows: P.E.N. affirms that: 1. Literature knows no frontiers and must remain common currency among people in spite of political or international upheavals. 2. In all circumstances, and particularly in time of war, works of art, the patrimony of humanity at large, should be left untouched by national or political passion. 3. Members of P.E.N. should at all times use what influence they have in favour of good understanding and mutual respect between nations; they pledge themselves to do their utmost to dispel race, class and national hatreds, and to champion the ideal of one humanity living in peace in one world. 4. P.E.N. stands for the principle of unhampered transmission of thought within each nation and between all nations, and members pledge themselves to oppose any form of suppression of freedom of expression in the country and community to which they belong, as well as throughout the world wherever this is possible. P.E.N. declares for a free press and opposes arbitrary censorship in time of peace. It believes that the necessary advance of the world towards a more highly organized political and economic order renders a free criticism of governments, administrations and institutions imperative.