Diversity and Productivity of Biotic Communities in Relation to Freshwater Inputs in Three Eastern Cape Estuaries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Presence, Levels and Distribution of Pollutants in the Estuarine Food Web- Swartkops River Estuary, South Africa
Presence, levels and distribution of pollutants in the estuarine food web- Swartkops River Estuary, South Africa L Nel 21250642 Dissertation submitted in fulfillment of the requirements for the degree Magister Scientiae in Zoology at the Potchefstroom Campus of the North-West University Supervisor: Prof H Bouwman Co-supervisor: Dr N Strydom September 2014 1 “Man can hardly even recognize the devils of his own creation” ~ Albert Schweitzer i Presence, levels and distribution of pollutants in the estuarine food web- Swartkops River Estuary, South Africa Acknowledgements The completion of this dissertation would not have been possible without the help and support from a number of people. To each who played a role, I want to personally thank you. To my parents, Pieter and Monique Nel, there is not enough ways to say thank you for the support, inspiration and unconditional love, for always being there and having the faith to see this through when I was no longer able to. The assistance and advice I have received from my supervisors Prof Henk Bouwman and Dr Nadine Strydom. Thank you for your guidance, patience and valuable contributions. To Anthony Kruger and Edward Truter who assisted with the collection of the fish. Your generosity and assistance was unbelievable and without you, I would be nowhere near complete. To Sabina Philips who helped and assisted throughout the time I was in Port Elizabeth. Your help, kindness and friendship are greatly appreciated. Paula Pattrick who took the time to help with the seine nets for the collection of smaller fish. To Deon Swart for the arrangement of the collecting permits and dealing with difficult authorities To my friends and family for their trust, support and encouragement. -

7. Impofu Grid Connection Rev Paleontology Report.Pdf
1 PALAEONTOLOGICAL HERITAGE: COMBINED DESKTOP & FIELD-BASED BASIC ASSESSMENT Grid connection for the proposed Impofu North, Impofu West & Impofu East Wind Farms near Humansdorp, Eastern Cape John E. Almond PhD (Cantab.) Natura Viva cc, PO Box 12410 Mill Street, Cape Town 8010, RSA [email protected] August 2019 EXECUTIVE SUMMARY The present report provides a palaeontological heritage Basic Assessment of the proposed Impofu grid connection. This includes (a) the approximately 120 km-long, 2 km-wide 132 kV grid connection corridor between the proposed Impofu North, Impofu West and Impofu East Wind Farms and the national grid in the Nelson Mandela Bay Municipality (NMBM) near Port Elizabeth, Eastern Cape. Potential impacts of the proposed new Impofu collector switching station, wind farm switching stations and short 132 kV transmission lines linking them to the collector switching station as well as of substation extension areas are also considered. The report is based on a combined desktop and field-based study of the preferred grid connection corridor, incorporating a 2 km wide zone, with a special focus on areas underlain by potentially fossiliferous bedrocks. The grid connection study area is underlain by several formations of potentially fossiliferous sediments of the Gamtoos Group, Cape Supergroup, Uitenhage Group and Algoa Group (Sections 6 & 7, Table 1). However, on the southern coastal platform most of the fossils originally preserved in these bedrocks appear to have been destroyed by tectonic deformation and deep chemical weathering. The overlying Late Caenozoic superficial sediments such as alluvium, soils and ferricretes, are likewise of low palaeontological sensitivity. Relict patches of Plio-Pleistocene aeolianites (wind-blown sands) of the Nanaga Formation (Algoa Group) present in the subsurface on the interior coastal platform contain Early Stone Age artefacts but any associated fossils such as mammalian remains, or terrestrial gastropods have probably been destroyed by weathering here. -
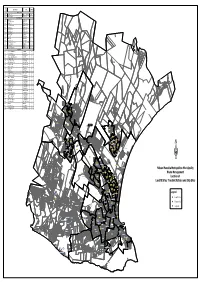
Map of Landfill Transfer and Skip Sites
Origin DESCRIPTION AREA WARD Code LANDFILL SITES G01 ARLINGTON PORT ELIZABETH 01 G02 KOEDOESKLOOF UITENHAGE 53 TRANSFER SITES G03 TAMBO STR McNAUGHTON 50 G04 GILLESPIE STR EXT UITENHAGE 53 G05 JOLOBE STR KWANOBUHLE 47 G06 ZOLILE NOGCAZI RD KWANOBUHLE 45 G07 SARILI STR KWANOBUHLE 42 G08 VERWOERD RD UITENHAGE 51 G09 NGEYAKHE STR KWANOBUHLE 42 G10 NTAMBANANI STR KWANOBUHLE 47 G11 LAKSMAN AVE ROSEDALE 49 G12 RALO STR KWAMAGXAKI 30 G13 GAIL ROAD GELVANDALE 13 G14 DITCHLING STR ALGOA PARK 11 G15 CAPE ROAD - OPP HOTEL HUNTERS RETREAT 12 G16 KRAGGAKAMA RD FRAMESBY 09 G17 5TH AVENUE WALMER 04 G18 STRANDFONTEIN RD SUMMERSTRAND 02 SKIP SITES G19 OFF WILLIAM MOFFAT FAIRVIEW 06 G20 STANFORD ROAD HELENVALE 13 G21 OLD UITENHAGE ROAD GOVAN MBEKI 33 G22 KATOO & HARRINGTON STR SALTLAKE 32 G23 BRAMLIN STREET MALABAR EXT 6 09 G24 ROHLILALA VILLAGE MISSIONVALE 32 G25 EMPILWENI HOSPITAL NEW BRIGHTON 31 G26 EMLOTHENI STR NEW BRIGHTON 14 G27 GUNGULUZA STR NEW BRIGHTON 16 G28 DAKU SQUARE KWAZAKHELE 24 G29 DAKU SPAR KWAZAKHELE 21 G30 TIPPERS CREEK BLUEWATER BAY 60 G31 CARDEN STR - EAST OF RAILWAY CROSSING REDHOUSE 60 G32 BHOBHOYI STR NU 8 MOTHERWELL 56 G33 UMNULU 1 STR NU 9 MOTHERWELL 58 Farms Uitenhage G34 NGXOTWANE STR NU 9 MOTHERWELL 57 G51 ward 53 S G35 MPONGO STR NU 7 MOTHERWELL 57 G36 MGWALANA STR NU 6 MOTHERWELL 59 G37 DABADABA STR NU 5 MOTHERWELL 59 G38 MBOKWANE NU 10 MOTHERWELL 55 Colchester G39 GOGO STR NU 1 MOTHERWELL 56 G40 MALINGA STR WELLS ESTATE 60 G41 SAKWATSHA STR NU 8 MOTHERWELL 58 G42 NGQOKWENI STR NU 9 MOTHERWELL 57 G43 UMNULU 2 STR NU 9 MOTHERWELL -

Part 17. the Cephalaspidea, Anaspidea, Pleurobranchida, and Sacoglossa (Mollusca: Gastropoda: Heterobranchia)
Archiv für Molluskenkunde 147 (1) 1–48 Frankfurt am Main, 29 June 2018 Results of the Rumphius Biohistorical Expedition to Ambon (1990). Part 17. The Cephalaspidea, Anaspidea, Pleurobranchida, and Sacoglossa (Mollusca: Gastropoda: Heterobranchia) Nathalie Yonow1 & Kathe R. Jensen2 1 Swansea Ecology Research Team, Department of Biosciences, Swansea University, Singleton Park, Swansea SA2 8PP, Wales, United Kingdom ([email protected]). 2 Zoological Museum, Universitetsparken 15, DK-2100 Copenhagen, Denmark. • Corresponding author: N. Yonow. Abstract. This is the final report describing the heterobranch molluscs collected by the Rumphius Bio historical Expedition to Ambon during November and December 1990. This part deals with the non‑nudibranch higher clades (Cephalaspidea, Anaspidea, Sacoglossa, and Pleurobranchida). Forty‑one species belonging to 21 genera are identified from Ambon and nearby localities in the central Indonesian archipelago; 2 species are new to science, and 3 species are new records for Indonesia. The new species Haminoea edmundsi is described from the mangroves near Paso in Ambon Bay, and the new species Mourgona anisti is described from Latuhalat, also Ambon. A discussion and table of all named species of Plakobranchus are provided, and the original descriptions and museum specimens from other localities are compared in an effort to clarify some of the problems surrounding these species. Chelidonura sandrana Rudman, 1973 and C. babai Gosliner, 1988 are synonymised with C. tsurugensis Baba & Abe, 1959. Recent corrections of names and/or dates of publication are highlighted: Phanerophthalmus olivaceus (Ehrenberg, 1828), Aplysia argus Rüppell & Leuckart, 1830, Dolabella auricularia ([Lightfoot], 1786), Elysia faustula Bergh, 1871, Cyerce elegans Bergh, 1870, Euselenops luniceps (Cuvier, 1816), and Pleurobranchus peronii Cuvier, 1804. -

The Hydrogeology of the Uitenhage Artesian Basin with Reference to the Table Mountain Group Aquifer
The hydrogeology of the Uitenhage Artesian Basin with reference to the Table Mountain Group Aquifer LGA Maclear SRK Consulting Engineers and Scientists, PO Box 21842, Port Elizabeth 6000, South Africa Abstract The Uitenhage Artesian Basin (UAB) lies in the Eastern Cape and is South Africa’s most important artesian groundwater basin, supplying approximately 1 400 Ml/yr (44 l/s) of water from springs for domestic use to Uitenhage, as well as supporting large citrus irrigation schemes. Groundwater from this basin has been extensively utilised from the early part of the 20th century, including periods of over-exploitation resulting in the declaration of a groundwater control area to limit abstraction to sustainable rates. The aquifer comprises fractured Table Mountain Group (TMG) sandstones confined in the eastern part of the basin by overlying Cretaceous siltstones and mudstones, resulting in artesian conditions. The Coega Fault is a major structural feature dividing the basin into separate systems, viz. the southern Swartkops Aquifer and the northern Coega Ridge Aquifer, that are hydrogeologically independent of each other. The Elands River syncline divides the Swartkops Aquifer further into the Kruisrivier and Bethelsdorp Units. Borehole yields commonly range from 5 to 10 l/s and the groundwater quality is excellent with low salinities. Water hardening, however, is required due to the acidic and corrosive nature of the groundwater, typical of other Table Mountain Group aquifers in South Africa. Using 14C data, the age of the groundwater in the basin ranges from 1 500 to 28 000 years with a calculated flow rate of 0.8 m/a. -

Spawning and Development of Some Hawaiian Marine Gastropods! JENS MATHIAS OSTERGAARD 2
Spawning and Development of Some Hawaiian Marine Gastropods! JENS MATHIAS OSTERGAARD 2 MANY PERSONS, at various marine biological lead pencil by the author from observation stations situated throughout the world, hav« under low power of the . compound micro investigated the spawning habits and larval scope. A micrometer scale was used so that development of marine gastropods. Some of measurements in fractions of. millimeters the recorded observations date back to the could be made readily. middle part of the last century or earlier. All the species investigated were obtained Various methods have been employed to ob in water not over 3 meters in depth, and most tain the materials for study, but always the of the egg structures were deposited in the effort put forth has been well rewarded, for laboratory by isolated animals. · When the the field is exceedingly rich. -It is noteworthy spawns were obtained from the reefs, or else that the study of plankton alone has been where, careful checks had been made to as able to throw much light 011 the metamor certain their identity, which in most cases phosis of the larvae of many species. This is was later verified. No record of any species a field of investigation that has an economic whose generic .position was unknown has as well as an academic value, inasmuch as been included. Hence, discussion of a con these minute planktonic larvae make a con siderable number of additional species of the siderable contribution to the food supply of Nudibranchiata, whose systematic position larger organisms, which in turn may serve has not yet been fully determined, awaits as food for fishes that are of direct economic later publication. -

A Polyvalent and Universal Tool for Genomic Studies In
A polyvalent and universal tool for genomic studies in gastropod molluscs (Heterobranchia: Tectipleura) Juan Moles1 and Gonzalo Giribet1 1Harvard University Faculty of Arts and Sciences April 28, 2020 Abstract Molluscs are the second most diverse animal phylum and heterobranch gastropods present ~44,000 species. These comprise fascinating creatures with a huge morphological and ecological disparity. Such great diversity comes with even larger phyloge- netic uncertainty and many taxa have been largely neglected in molecular assessments. Genomic tools have provided resolution to deep cladogenic events but generating large numbers of transcriptomes/genomes is expensive and usually requires fresh material. Here we leverage a target enrichment approach to design and synthesize a probe set based on available genomes and transcriptomes across Heterobranchia. Our probe set contains 57,606 70mer baits and targets a total of 2,259 ultra-conserved elements (UCEs). Post-sequencing capture efficiency was tested against 31 marine heterobranchs from major groups, includ- ing Acochlidia, Acteonoidea, Aplysiida, Cephalaspidea, Pleurobranchida, Pteropoda, Runcinida, Sacoglossa, and Umbraculida. The combined Trinity and Velvet assemblies recovered up to 2,211 UCEs in Tectipleura and up to 1,978 in Nudipleura, the most distantly related taxon to our core study group. Total alignment length was 525,599 bp and contained 52% informative sites and 21% missing data. Maximum-likelihood and Bayesian inference approaches recovered the monophyly of all orders tested as well as the larger clades Nudipleura, Panpulmonata, and Euopisthobranchia. The successful enrichment of diversely preserved material and DNA concentrations demonstrate the polyvalent nature of UCEs, and the universality of the probe set designed. We believe this probe set will enable multiple, interesting lines of research, that will benefit from an inexpensive and largely informative tool that will, additionally, benefit from the access to museum collections to gather genomic data. -

A New Phylogeny of the Cephalaspidea (Gastropoda: Heterobranchia) Based on Expanded Taxon Sampling and Gene Markers Q ⇑ Trond R
Molecular Phylogenetics and Evolution 89 (2015) 130–150 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A new phylogeny of the Cephalaspidea (Gastropoda: Heterobranchia) based on expanded taxon sampling and gene markers q ⇑ Trond R. Oskars a, , Philippe Bouchet b, Manuel António E. Malaquias a a Phylogenetic Systematics and Evolution Research Group, Section of Taxonomy and Evolution, Department of Natural History, University Museum of Bergen, University of Bergen, PB 7800, 5020 Bergen, Norway b Muséum National d’Histoire Naturelle, UMR 7205, ISyEB, 55 rue de Buffon, F-75231 Paris cedex 05, France article info abstract Article history: The Cephalaspidea is a diverse marine clade of euthyneuran gastropods with many groups still known Received 28 November 2014 largely from shells or scant anatomical data. The definition of the group and the relationships between Revised 14 March 2015 members has been hampered by the difficulty of establishing sound synapomorphies, but the advent Accepted 8 April 2015 of molecular phylogenetics is helping to change significantly this situation. Yet, because of limited taxon Available online 24 April 2015 sampling and few genetic markers employed in previous studies, many questions about the sister rela- tionships and monophyletic status of several families remained open. Keywords: In this study 109 species of Cephalaspidea were included covering 100% of traditional family-level Gastropoda diversity (12 families) and 50% of all genera (33 genera). Bayesian and maximum likelihood phylogenet- Euthyneura Bubble snails ics analyses based on two mitochondrial (COI, 16S rRNA) and two nuclear gene markers (28S rRNA and Cephalaspids Histone-3) were used to infer the relationships of Cephalaspidea. -

A Check List of Opisthobranch Snails of the Karachi Coast
A check list of opisthobranch snails of the Karachi coast Item Type article Authors Kazmi, Quddusi B.; Tirmizi, Nasima M.; Zehra, Itrat Download date 01/10/2021 23:01:17 Link to Item http://hdl.handle.net/1834/33270 Pakistan Journal of Marine Sciences, Vol.5(1), 69-104, 1996. A CHECK LIST OF OPISTHOBRANCH SNAILS OF THE KARACID COAST Quddusi B.Kazmi, Nasima M.Tirmizi and Itrat Zebra Marine Reference Collection and Resource Centre(QBK; NMT); Centre of Excellence in Marine Biology (IZ), University of Karachi, Karachi-75270, Pakistan ABSTRACT: The check list deals with 44 species of opisthobranchs belonging to Cephalaspidea (12 species), Ahaspidea (4 species), Sacoglossa (4 species), Notaspidea, (2 species) and N~ibranchia (21 species), collect ed from Pakistan coast of northern Arabian Sea. KEY WORDS: Sea slugs, Karachi, Sindh coast, check list INTRODUCTION Earlier studies on the opisthobranch molluscan fauna of Karachi - Sindh coast were made by Eliot (1905) and Khan et al. (1971, 1973) reporting only a few species. Since reports by Woodwards (1856), Murray (1887) and Sowerby (1895) and not avail able nothing can be said relevant to opisthobranchs in these works. One order Thocosomata of this subclass was treated by Frontier (1963), Fatima (1988) and Zebra & Nayeem (199:5); this order is not incorporated here due to its planktonic exis tence. Tirmizi (1977, restricted) dealt with only few species of opisthobranchs and other molluscs. Tirmizi & Zebra (1982) gave in their generic key some opisthobranchs, later however only two species were reported in a monographic work on gastropods (Tirmizi & Zebra, 1984). Some work has been done on the reproductive biology (Zebra et al.,l988, 1995); from the HEJ Research Institute of Chemistry, University of Karachi biochemical substances from an anaspid Aplysia juliana are reported (Rahman, et al., 1991), the specimens are not seen by us. -

From the Marshall Islands, Including 57 New Records 1
Pacific Science (1983), vol. 37, no. 3 © 1984 by the University of Hawaii Press. All rights reserved Notes on Some Opisthobranchia (Mollusca: Gastropoda) from the Marshall Islands, Including 57 New Records 1 SCOTT JOHNSON2 and LISA M. BOUCHER2 ABSTRACT: The rich opisthobranch fauna of the Marshall Islands has re mained largely unstudied because of the geographic remoteness of these Pacific islands. We report on a long-term collection ofOpisthobranchia assembled from the atolls of Bikini, Enewetak, Kwajalein, Rongelap, and Ujelang . Fifty-seven new records for the Marshall Islands are recorded, raising to 103 the number of species reported from these islands. Aspects ofthe morphology, ecology, devel opment, and systematics of 76 of these species are discussed. THE OPISTHOBRANCH FAUNA OF THE Marshall viously named species are discussed, 57 of Islands, a group of 29 atolls and five single which are new records for the Marshall islands situated 3500 to 4400 km west south Islands (Table 1). west of Honolulu, Hawaii, is rich and varied but has not been reported on in any detail. Pre vious records of Marshall Islands' Opistho METHODS branchia record only 36 species and are largely restricted to three studies. Opisthobranchs The present collections were made on inter collected in the northern Marshalls during the tidal reefs and in shallow water by snorkeling period of nuclear testing (1946 to 1958) and and by scuba diving to depths of 25 m, both now in the U.S. National Museum, along with by day and night. additional material from Micronesia, were Descriptions, measurements, and color studied by Marcus (1965). -

The Bubble Snails (Gastropoda, Heterobranchia) of Mozambique: an Overlooked Biodiversity Hotspot
Mar Biodiv DOI 10.1007/s12526-016-0500-7 ORIGINAL PAPER The bubble snails (Gastropoda, Heterobranchia) of Mozambique: an overlooked biodiversity hotspot Yara Tibiriçá1,2 & Manuel António E. Malaquias3 Received: 9 October 2015 /Revised: 14 March 2016 /Accepted: 17 April 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract This first account, dedicated to the shallow water Keywords Mollusca . Acteonoidea . Cephalaspidea . Sea marine heterobranch gastropods of Mozambique is presented slugs . Taxonomy . Biodiversity . Africa . Western Indian with a focus on the clades Acteonoidea and Cephalaspidea. Ocean Specimens were obtained as a result of sporadic sampling and two dedicated field campaigns between the years of 2012 and 2015, conducted along the northern and southern coasts of Introduction Mozambique. Specimens were collected by hand in the inter- tidal and subtidal reefs by snorkelling or SCUBA diving down The shallow water marine heterobranch gastropods (sea slugs to a depth of 33 m. Thirty-two species were found, of which and alikes) of the Western Indian Ocean (WIO), an area con- 22 are new records to Mozambique and five are new for the fined between the East African coast and the Saya de Malha, Western Indian Ocean. This account raises the total number of Nazareth, and Cargados Carajos banks of the Mascarene shallow water Acteonoidea and Cephalaspidea known in Plateau (Obura 2012), have received little attention when Mozambique to 39 species, which represents approximately compared to other parts of the Indo-West Pacific, and most 50 % of the Indian Ocean diversity and 83 % of the diversity accounts are relatively old with few recent contributions (e.g. -

Survey of Heavy Metals in the Sediments of the Swartkops River Estuary, Port Elizabeth South Africa
Survey of heavy metals in the sediments of the Swartkops River Estuary, Port Elizabeth South Africa Karen Binning and Dan Baird* Zoology Department, University of Port Elizabeth, PO Box 1600, Port Elizabeth 6000, South Africa Abstract Elevated levels of heavy metals in the sediment can be a good indication of man-induced pollution. Concentrations of chrome, lead, zinc, titanium, manganese, strontium, copper and tin were measured in the sediments taken along a section of the Swartkops River and its estuary. These results showed that the highest heavy metal concentrations in both the estuary and river were recorded at points where runoff from informal settlements and industry entered the system. Comparison of the results for the estuary with those obtained in a similar survey made about 20 years ago revealed some remarkable increases. This raises concern over the long-term health of the Swartkops River ecosystem. Introduction pollution in a particular region or ecosystem. Heavy metal concentrations in the water column can be relatively low, but the The Swartkops River flows through a highly urbanised and concentrations in the sediment may be elevated. Low level discharges industrialised region of the Eastern Cape and forms an integral part of a contaminant may meet the water quality criteria, but long-term of Port Elizabeth and the surrounding areas. It is a valuable partitioning to the sediments could result in the accumulation of recreational and ecological asset, but owing to the rapidly expanding high loads of pollutants. It has been estimated that about 90% of urban areas, it is subject to the effects and influences of these particulate matter carried by rivers settles in estuaries and coastal developments.