Table S1 the Important Researches About Rapoport's Rule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seidenfaden Malaysia: 0.65 These Figures Are Surprisingly High, They Apply to Single Only. T
BIOGEOGRAPHY OF MALESIAN ORCHIDACEAE 273 VIII. Biogeographyof Malesian Orchidaceae A. Schuiteman Rijksherbarium/Hortus Botanicus, P.O. Box 9514, 2300 RA Leiden, The Netherlands INTRODUCTION The Orchidaceae outnumber far other in Malesia. At how- by any plant family present, accurate estimate of the of Malesian orchid is difficult to make. ever, an number species Subtracting the numberofestablishedsynonyms from the numberof names attributed to Malesian orchid species results in the staggering figure of 6414 species, with a retention of 0.74. This is ratio (ratio of ‘accepted’ species to heterotypic names) undoubtedly a overestimate, of the 209 Malesian orchid have been revised gross as most genera never their entire from availablerevisions estimate realis- over range. Extrapolating to a more tic retention ratio is problematic due to the small number of modern revisions and the different of treated. If look for Malesian of nature the groups we comparison at species wide ofretention ratios: some recently revised groups, we encounter a range Bulbophylluw sect. Uncifera (Vermeulen, 1993): 0.24 Dendrobium sect. Oxyglossum (Reeve & Woods, 1989): 0.24 Mediocalcar (Schuiteman, 1997): 0.29 Pholidota (De Vogel, 1988): 0.29 Bulbophyllum sect. Pelma (Vermeulen, 1993): 0.50 Paphiopedilum (Cribb, 1987, modified): 0.57 Dendrobium sect. Spatulata (Cribb, 1986, modified): 0.60. Correspondingly, we find a wide rangeof estimates for the ‘real’ numberof known Male- sian orchid species: from 2050 to 5125. Another approach would be to look at a single area, and to compute the retention ratio for the orchid flora of that area. If we do this for Java (mainly based on Comber, 1990), Peninsular Malaysia & Singapore (Seidenfaden & Wood, 1992) and Sumatra (J.J. -

A Monograph of the Genus Microtoena (Lamiaceae) This Book Was Originally Published by Science Press, © Science Press, 2018
Current Natural Sciences WANG Q. Current Natural Sciences BOTANY A Monograph of the Genus BOTANY Microtoena (Lamiaceae) Qiang WANG The genus Microtoena is a lovely and enigmatic member of the mint family. This new monograph is a comprehensive taxonomic revision, based on extensive field observations, population of the Genus A Monograph sampling, specimen examinations, scanning electron microscopy observations, statistical analysis of characters, and molecular phylogenetic analysis. Detailed species descriptions are Qiang WANG enhanced by 20 maps, 18 full page line drawings and numerous field photos. This is an essential reference for taxonomists, growers, breeders and horticulturalists. Qiang WANG. Associate Professor at the State Key Laboratory of systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences. A young taxonomist, his main interests A Monograph of the Genus are taxonomy, phylogeny and evolution of Lamiaceae, and his Microtoena passion for Microtoena stretches back 10 years. Microtoena (Lamiaceae) (Lamiaceae) ISBN : 978-2-7598-2528-8 www.edpsciences.org Price: 95 € 9 782759 825288 Current Natural Sciences WANG Q. Current Natural Sciences BOTANY A Monograph of the Genus BOTANY Microtoena (Lamiaceae) Qiang WANG The genus Microtoena is a lovely and enigmatic member of the mint family. This new monograph is a comprehensive taxonomic revision, based on extensive field observations, population of the Genus A Monograph sampling, specimen examinations, scanning electron microscopy observations, statistical analysis of characters, and molecular phylogenetic analysis. Detailed species descriptions are Qiang WANG enhanced by 20 maps, 18 full page line drawings and numerous field photos. This is an essential reference for taxonomists, growers, breeders and horticulturalists. Qiang WANG. -

Epilist 1.0: a Global Checklist of Vascular Epiphytes
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2021 EpiList 1.0: a global checklist of vascular epiphytes Zotz, Gerhard ; Weigelt, Patrick ; Kessler, Michael ; Kreft, Holger ; Taylor, Amanda Abstract: Epiphytes make up roughly 10% of all vascular plant species globally and play important functional roles, especially in tropical forests. However, to date, there is no comprehensive list of vas- cular epiphyte species. Here, we present EpiList 1.0, the first global list of vascular epiphytes based on standardized definitions and taxonomy. We include obligate epiphytes, facultative epiphytes, and hemiepiphytes, as the latter share the vulnerable epiphytic stage as juveniles. Based on 978 references, the checklist includes >31,000 species of 79 plant families. Species names were standardized against World Flora Online for seed plants and against the World Ferns database for lycophytes and ferns. In cases of species missing from these databases, we used other databases (mostly World Checklist of Selected Plant Families). For all species, author names and IDs for World Flora Online entries are provided to facilitate the alignment with other plant databases, and to avoid ambiguities. EpiList 1.0 will be a rich source for synthetic studies in ecology, biogeography, and evolutionary biology as it offers, for the first time, a species‐level overview over all currently known vascular epiphytes. At the same time, the list represents work in progress: species descriptions of epiphytic taxa are ongoing and published life form information in floristic inventories and trait and distribution databases is often incomplete and sometimes evenwrong. -

Trees, Shrubs, and Perennials That Intrigue Me (Gymnosperms First
Big-picture, evolutionary view of trees and shrubs (and a few of my favorite herbaceous perennials), ver. 2007-11-04 Descriptions of the trees and shrubs taken (stolen!!!) from online sources, from my own observations in and around Greenwood Lake, NY, and from these books: • Dirr’s Hardy Trees and Shrubs, Michael A. Dirr, Timber Press, © 1997 • Trees of North America (Golden field guide), C. Frank Brockman, St. Martin’s Press, © 2001 • Smithsonian Handbooks, Trees, Allen J. Coombes, Dorling Kindersley, © 2002 • Native Trees for North American Landscapes, Guy Sternberg with Jim Wilson, Timber Press, © 2004 • Complete Trees, Shrubs, and Hedges, Jacqueline Hériteau, © 2006 They are generally listed from most ancient to most recently evolved. (I’m not sure if this is true for the rosids and asterids, starting on page 30. I just listed them in the same order as Angiosperm Phylogeny Group II.) This document started out as my personal landscaping plan and morphed into something almost unwieldy and phantasmagorical. Key to symbols and colored text: Checkboxes indicate species and/or cultivars that I want. Checkmarks indicate those that I have (or that one of my neighbors has). Text in blue indicates shrub or hedge. (Unfinished task – there is no text in blue other than this text right here.) Text in red indicates that the species or cultivar is undesirable: • Out of range climatically (either wrong zone, or won’t do well because of differences in moisture or seasons, even though it is in the “right” zone). • Will grow too tall or wide and simply won’t fit well on my property. -

Lamiales – Synoptical Classification Vers
Lamiales – Synoptical classification vers. 2.6.2 (in prog.) Updated: 12 April, 2016 A Synoptical Classification of the Lamiales Version 2.6.2 (This is a working document) Compiled by Richard Olmstead With the help of: D. Albach, P. Beardsley, D. Bedigian, B. Bremer, P. Cantino, J. Chau, J. L. Clark, B. Drew, P. Garnock- Jones, S. Grose (Heydler), R. Harley, H.-D. Ihlenfeldt, B. Li, L. Lohmann, S. Mathews, L. McDade, K. Müller, E. Norman, N. O’Leary, B. Oxelman, J. Reveal, R. Scotland, J. Smith, D. Tank, E. Tripp, S. Wagstaff, E. Wallander, A. Weber, A. Wolfe, A. Wortley, N. Young, M. Zjhra, and many others [estimated 25 families, 1041 genera, and ca. 21,878 species in Lamiales] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near- term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA. -

New Orchid Taxa and Records in the Flora of Vietnam
Hội Hoa Lan Việt Nam www.hoalanvietnam.org/ New Orchid Taxa and Records in the Flora of Vietnam 19/07/2012 Dr. Leonid Averyanov ABSTRACT: Paper presents descriptions of 1 new genus (Theana), 4 new species (Theana vietnamica, Bulbophyllum salmoneum, Sarcoglyphis brevilabia, Schoenorchis scolopendria), 1 new variety (Dendrobium thyrsiflorum var. minutiflorum) and provides data on 21 species of orchids (Bulbophyllum bicolor, B. muscicola, B. nigrescens, B. putii, B. violaceolabellum, Calanthe mannii, C. whiteana, Coelogyne micrantha, Cymbidium cyperifolium, Dendrobium dixanthum, D. findlayanum, D. metrium, D. senile, Didymoplexiella ornata, Luisia thailandica, Monomeria gymnopus, Papilionanthe teres, Schoenorchis fragrans, Stereochilus brevirachis, S. erinaceus, Vanda brunnea) newly recorded in the flora of Vietnam. KEY WORDS: Nature protection, Orchidaceae, plant diversity, plant taxonomy, Vietnam. INTRODUCTION Paper contains results of modern field botanical explorations in eastern Indochina during last two years. It includes descriptions of 6 newly discovered taxa and data for 21 species discovered and recorded for Vietnam at first. Among them 1 new genus (Theana Aver.), 4 new species (Theana vietnamica Aver., Bulbophyllum salmoneum Aver. et J.J.Verm., Sarcoglyphis brevilabia Aver., Schoenorchis scolopendria Aver.), 1 new variety (Dendrobium thyrsiflorum var. minutiflorum Aver.) and 21 species discovered in Vietnam at first (Bulbophyllum bicolor Lindl., B. muscicola Rchb.f., B. nigrescens Rolfe, B. putii Seidenf., B. violaceolabellum Seidenf., Calanthe mannii Hook.f., C. whiteana King et Pantl., Coelogyne micrantha Lindl., Cymbidium cyperifolium Wall. ex Lindl., Dendrobium dixanthum Rchb.f., D. findlayanum C.S.P. Parish et Rchb.f., D. metrium Kraenzl., D. senile Parish et Rchb.f., Didymoplexiella ornata (Ridl.) Garay, Luisia thailandica Seidenf., Monomeria gymnopus (Hook.f.) Aver., Papilionanthe teres Schltr., Schoenorchis fragrans (C.S.P.Parish et Rchb.f.) Seidenf. -

Illustration Sources
APPENDIX ONE ILLUSTRATION SOURCES REF. CODE ABR Abrams, L. 1923–1960. Illustrated flora of the Pacific states. Stanford University Press, Stanford, CA. ADD Addisonia. 1916–1964. New York Botanical Garden, New York. Reprinted with permission from Addisonia, vol. 18, plate 579, Copyright © 1933, The New York Botanical Garden. ANDAnderson, E. and Woodson, R.E. 1935. The species of Tradescantia indigenous to the United States. Arnold Arboretum of Harvard University, Cambridge, MA. Reprinted with permission of the Arnold Arboretum of Harvard University. ANN Hollingworth A. 2005. Original illustrations. Published herein by the Botanical Research Institute of Texas, Fort Worth. Artist: Anne Hollingworth. ANO Anonymous. 1821. Medical botany. E. Cox and Sons, London. ARM Annual Rep. Missouri Bot. Gard. 1889–1912. Missouri Botanical Garden, St. Louis. BA1 Bailey, L.H. 1914–1917. The standard cyclopedia of horticulture. The Macmillan Company, New York. BA2 Bailey, L.H. and Bailey, E.Z. 1976. Hortus third: A concise dictionary of plants cultivated in the United States and Canada. Revised and expanded by the staff of the Liberty Hyde Bailey Hortorium. Cornell University. Macmillan Publishing Company, New York. Reprinted with permission from William Crepet and the L.H. Bailey Hortorium. Cornell University. BA3 Bailey, L.H. 1900–1902. Cyclopedia of American horticulture. Macmillan Publishing Company, New York. BB2 Britton, N.L. and Brown, A. 1913. An illustrated flora of the northern United States, Canada and the British posses- sions. Charles Scribner’s Sons, New York. BEA Beal, E.O. and Thieret, J.W. 1986. Aquatic and wetland plants of Kentucky. Kentucky Nature Preserves Commission, Frankfort. Reprinted with permission of Kentucky State Nature Preserves Commission. -

The Genus Koilodepas Hassk. (Euphorbiaceae) in Malesia
B I O D I V E R S I T A S ISSN: 1412-033X Volume 5, Nomor 2 Juli 2004 Halaman: 52-60 The Genus Koilodepas Hassk. (Euphorbiaceae) in Malesia MUZAYYINAH1,♥, EDI GUHARDJA2, MIEN A. RIFAI3, JOHANIS P. MOGEA3, PETER VAN WALZEN4 1Program Study of Biological Education, Department of PMIPA FKIP Sebelas Maret University Surakarta 57126, Indonesia. 2Department of Biology, Faculty of Mathematic and Sciences, Bogor Agriculture University, Bogor Indonesia 3"Herbarium Bogoriense", Department of Botany, Research Center of Biology -LIPI, Bogor 16013, Indonesia 4Rijksherbarium Leiden, The Netherlands. Received: 8th December 2003. Accepted: 17th May 2004. ABSTRACT The Malesian genus Koilodepas Hassk. has been revised based on the morphological and anatomical character using available herbarium collection in Herbarium Bogoriense and loan specimens from Kew Herbarium and Leiden Rijksherbarium. The present study is based on the observation of 176 specimens. Eight species has been recognized, namely K. bantamense, K. cordifolium, K. frutescns, K. homalifolium, K. laevigatum, K. longifolium, K. pectinatum, and two varieties within K. brevipes. The highest number of species is found in Borneo (5 species), three of them are found endemically in Borneo, K. cordisepalum endemically in Aceh, and K. homalifolium endemically in Papua New Guinea. A phylogenetic analysis of the genus, with Cephalomappa as outgroup, show that the species within the genus Koilodepas is in one group, starting with K. brevipes as a primitive one and K. bantamense occupies in an advance position. 2004 Jurusan Biologi FMIPA UNS Surakarta Keyword: Koilodepas Hassk., Malesia, outgroup, phylogenetic analysis, outgroup. INTRODUCTION Methods The methods of research were morphological and The genus Koilodepas belongs to the tribe Epipri- leaf-anatomical description. -

Key to the Families and Genera of Malesian <I>Euphorbiaceae</I> in the Wide Sense
Blumea 65, 2020: 53–60 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE https://doi.org/10.3767/blumea.2020.65.01.05 Key to the families and genera of Malesian Euphorbiaceae in the wide sense P.C. van Welzen1,2 Key words Abstract Identification keys are provided to the different families in which the Euphorbiaceae are split after APG IV. Presently, Euphorbiaceae in the strict sense, Pandaceae, Peraceae, Phyllanthaceae, Picrodendraceae and Euphorbiaceae Putranjivaceae are distinguished as distinct families. Within the families, keys to the different genera occurring in the keys Malesian area, native and introduced, are presented. The keys are to be tested and responses are very welcome. Pandaceae Peraceae Published on 3 April 2020 Phyllanthaceae Picrodendraceae Putranjivaceae INTRODUCTION KEY TO THE EUPHORBIACEOUS FAMILIES The Euphorbiaceae in the wide sense (sensu lato, s.lat.) were 1. Ovary with a single ovule per locule . 2 always a heterogeneous group without any distinct combination 1. Ovary with two ovules per locule ................... 4 of characters. The most typical features are the presence of uni- 2. Fruits drupes. Flowers of both sexes with petals . sexual simple flowers and fruits that fall apart in various carpel ...................................2. Pandaceae fragments and seeds, leaving the characteristic columella on the 2. Fruits capsules, sometimes drupes or berries, then flowers plant. However, some groups, like the Pandaceae and Putranji of both sexes lacking petals.......................3 vaceae, were morphologically already known to be quite unlike 3. Herbs, shrubs, lianas, trees, mono- or dioecious. Flowers in the rest of the Euphorbiaceae s.lat. (e.g., Radcliffe-Smith 1987). cauliflorous, ramiflorous, axillary, or terminal inflorescences The Putranjivaceae formerly formed the tribe Drypeteae in the ................................1. -

ONEP V08.Pdf
Compiled by Obchant Thaithong Office of Environmental Policy and Planning 1999 First published December 1999 by Office of Environmental Policy and Planning (OEPP), Thailand. ISBN : 974-7579-61-8 This publication is financially supported by OEPP and may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from OEPP, providing that acknowledgment of the source is made. No use of this publication may be made for resale or for any other commercial purposes. Citation : Obchant Thaithong, 1999. Orchids of Thailand. Office of Environmental Policy and Planning, Bangkok, Thailand. About the authors : Associate Professor Obchant Thaithong, Department of Botany, Faculty of Science, Chulalongkorn University. Available from : Biological Resources Section Natural Resources and Environmental Management Division Office of Environmental Policy and Planning Ministry of Science Technology and Environment 60/1 Rama VI Rd. Bangkok 10400 THAILAND Tel. (662) 2713251, 2797180, 2797186-9 ext 226, 227 Facsimile (662) 2798088, 2713251 Designed & Printed : Integrated Promotion Technology Co., Ltd. Tel. (662) 5852076, 5860837, 9137761-2 Facsimile (662) 9137763 2 Habitat (ถิ่นอาศัย) Epiphyte Epiphyte (ขนบนต้ึ นไม้ )้ (ขนบนต้ึ นไม้ )้ Lithophyte Terrestrial (ขนบนห้ึ นิ ) (ขนบนด้ึ นิ ) 11 Apostasioideae Apostasia nuda R. Br. Cypripedioideae Paphiopedilum godefroyae (Godefr.) Stein Paphiopedilum villosum Pfitz. Paphiopedilum concolor (Lindl.) Pfitz. 12 Neottioideae Anoectochilus burmanicus Rolfe. Corymborkis veratifolia (Reinw.) Bl. Goodyera procera (Ker. & Gawl.) Herpysma longicaulis Lindl. Hook. Ludisia discolor (Ker. & Gawl.) Didymoplexiopsis kiriwongensis A. Reich. Seidenf. 13 Orchidoideae Brachycorythis henryi (Schltr.) Summ. Habenaria chlorina Par. & Rchb. f. Habenaria rhodocheila Hance Habenaria lindleyana steudl. Pecteilis henryi Schltr. Peristylus lacertiferus (Lindl.) J.J.Sm. -
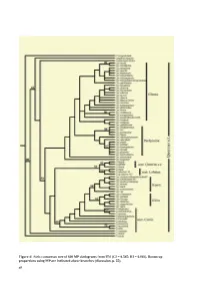
Taxonomy and Systematics of Quercus Subgenus Cyclobalanopsis
Figure 4/ Strict consensus tree of 600 MP cladograms from ITS (CI = 0.545; RI = 0.803). Bootstrap proportions using MP are indicated above branches (discussion, p. 55). 48 Taxonomy and Systematics of Quercus Subgenus Cyclobalanopsis Min Deng1, Zhekun Zhou2*, Qiansheng Li3 2. Xishuangbanna Tropical Botanical Garden, 3. School of Ecology, the Chinese Academy of Sciences, Shanghai Institute of Technology, Kunming, 650223, China. 100 Haiquan Rd., Shanghai, 201418, China 1. Chenshan Plant Sciences Research Center, the Chinese Academy of Sciences, Chenshan Botanical Garden, 3888 ChenHua Rd., Shanghai, 201602, China. Phone: +86-21- 57 79 93 82; Fax: + 86-21-67657811. [email protected]; ABSTRACT Quercus subgenus Cyclobalanopsis is one of the dominant woody plant groups in E and SE Asia, but comprehensive studies on its systematics and taxonomy are limited. In this study, we compared the leaf epidermal and acorn features of 52 species in subgenus Cyclobalanopsis and 15 species from Quercus subgenus Quercus. We also studied molecular phylogeny using DNA sequences from the nuclear ribosomal internal transcribed spacer (ITS) region and the chloroplast psbA–trnH and trnT–trnL regions. Both the leaf epidermal and acorn features indicated ʏve morphologically distinct groups in Cyclobalanopsis: 1) Gilva group (fused stellate trichomes with compound trichome base); 2) Kerrii group (with fasciculate trichomes, and radicles emerging from the basal seed scar; 3) Pachyloma group (papillae on epidermal cells, but glabrous when mature and densely yellowish woolly on the cupule walls); 4) Jenseniana group (lamellae mostly fused to the cupule wall with only the rims free); 5) Glauca group (appressed-lateral-attached trichomes). -

Accepted Manuscript
Diverse trajectories of plastome degradation in holoparasitic Cistanche and the whereabouts of the lost plastid genes Downloaded from https://academic.oup.com/jxb/advance-article-abstract/doi/10.1093/jxb/erz456/5602659 by Harvard Library user on 01 November 2019 Xiaoqing Liu1, Weirui Fu1, Yiwei Tang1, Wenju Zhang1, Zhiping Song1, Linfeng Li1, Ji Yang1, Hong Ma2,3, Jianhua Yang4, Chan Zhou5, Charles C. Davis6 and Yuguo Wang1,† 1 Ministry of Education Key Laboratory for Biodiversity Science and Ecological Engineering, Institute of Biodiversity Science, School of Life Sciences, Fudan University, Shanghai 200433, China 2 State Key Laboratory of Genetic Engineering, School of Life Sciences, Institute of Plant Biology, Center for Evolutionary Biology, Fudan University, Shanghai 200433, China 3 Department of Biology, Institute of Molecular Evolutionary Genetics, and the Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, Pennsylvania 16802, USA 4 College of Pharmacy, The First Affiliated Hospital, Xinjiang Medical University, Urumqi 830011, China 5 Department of Population and Quantitative Health Sciences, Massachusetts General Hospital, 55 Lake Ave North Worcester, MA 01605, USA 6 Department of Organismic and Evolutionary Biology, Harvard University Herbaria, 22 Divinity Avenue, Cambridge, MA 02138, USA † Correspondence: [email protected]; Tel: 0086-21-31246697 Accepted Manuscript The email address for each author: Xiaoqing Liu, [email protected]; Weirui Fu, [email protected]; Yiwei Tang,