Predicting Nutrient Content of Ray-Finned Fishes Using
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LONGFIN SMELT Spirinchus Thaleichthys USFWS: None CDFG: Threatened
LSA ASSOCIATES, INC. PUBLIC DRAFT SOLANO HCP JULY 2012 SOLANO COUNTY WATER AGENCY NATURAL COMMUNITY AND SPECIES ACCOUNTS LONGFIN SMELT Spirinchus thaleichthys USFWS: None CDFG: Threatened Species Account Status and Description. The longfin smelt is listed as a threatened species by the California Fish and Game Commission. Abundance of the longfin smelt has reached record lows in the San Francisco-Delta population, and the species may already be extinct in some northern California estuarine populations, resulting in an overall threat of extinction to the species within California (Federal Register 2008). The longfin smelt was also proposed for federal listing, but on April 8, 2009 the USFWS determined that the San Francisco Bay Estuary population does not qualify for listing as a distinct population segment under federal regulations. Further assessment of the entire population is being conducted, however, and future listing may be considered. Photo courtesy of California Department of Fish and Game Longfin smelt, once mature, are slim, silver fish in the family Osmeridae (true smelts). Moyle (2002) describes the species as being 90-110 mm (standard length) at maturity, with a translucent silver appearance along the sides of the body, and an olive to iridescent pinkish hue on the back. Mature males are often darker than females, with enlarged and stiffened dorsal and anal fins, a dilated lateral line region, and breeding tubercles on paired fins and scares. Longfin smelt can be distinguished from other California smelt by their long pectoral fins (which reach or nearly reach the bases of the pelvic fins), incomplete lateral line, weak or absent striations on the opercular bones, low number of scales in the lateral line (54-65) and long maxillary bones (which in adults extent just short of the posterior margin of the eye). -

Cottus Poecilopus Heckel, 1836, in the River Javorin- Ka, the Tatra
Oecologia Montana 2018, Cottus poecilopus Heckel, 1836, in the river Javorin- 27, 21-26 ka, the Tatra mountains, Slovakia M. JANIGA, Jr. In Tatranská Javorina under Muráň mountain, a small fish nursery was built by Christian Kraft von Institute of High Mountain Biology University of Hohenlohe around 1930. The most comprehensive Žilina, Tatranská Javorina 7, SK-059 56, Slovakia; studies on fish from the Tatra mountains were writ- e-mail:: [email protected] ten by professor Václav Dyk (1957; 1961), Dyk and Dyková (1964a,b; 1965), who studied altitudinal distribution of fish, describing the highest points where fish were found. His studies on fish were likely the most complex studies of their kind during that period. Along with his wife Sylvia, who illus- Abstract. This study focuses on the Cottus poe- trated his studies, they published the first realistic cilopus from the river Javorinka in the north-east studies on fish from the Tatra mountains including High Tatra mountains, Slovakia. The movement the river Javorinka (Dyk and Dyková 1964a). Feri- and residence of 75 Alpine bullhead in the river anc (1948) published the first Slovakian nomenclature were monitored and carefully recorded using GPS of fish in 1948. Eugen K. Balon (1964; 1966) was the coordinates. A map representing their location in next famous ichthyologist who became a recognised the river was generated. This data was collected in expert in the fish fauna of the streams of the Tatra the spring and summer of 2016 and in the autumn mountains, the river Poprad, and various high moun- of 2017. Body length and body weight of 67 Alpine tain lakes. -

Forage Fishes of the Southeastern Bering Sea Conference Proceedings
a OCS Study MMS 87-0017 Forage Fishes of the Southeastern Bering Sea Conference Proceedings 1-1 July 1987 Minerals Management Service Alaska OCS Region OCS Study MMS 87-0017 FORAGE FISHES OF THE SOUTHEASTERN BERING SEA Proceedings of a Conference 4-5 November 1986 Anchorage Hilton Hotel Anchorage, Alaska Prepared f br: U.S. Department of the Interior Minerals Management Service Alaska OCS Region 949 East 36th Avenue, Room 110 Anchorage, Alaska 99508-4302 Under Contract No. 14-12-0001-30297 Logistical Support and Report Preparation By: MBC Applied Environmental Sciences 947 Newhall Street Costa Mesa, California 92627 July 1987 CONTENTS Page ACKNOWLEDGMENTS .............................. iv INTRODUCTION PAPERS Dynamics of the Southeastern Bering Sea Oceanographic Environment - H. Joseph Niebauer .................................. The Bering Sea Ecosystem as a Predation Controlled System - Taivo Laevastu .... Marine Mammals and Forage Fishes in the Southeastern Bering Sea - Kathryn J. Frost and Lloyd Lowry. ............................. Trophic Interactions Between Forage Fish and Seabirds in the Southeastern Bering Sea - Gerald A. Sanger ............................ Demersal Fish Predators of Pelagic Forage Fishes in the Southeastern Bering Sea - M. James Allen ................................ Dynamics of Coastal Salmon in the Southeastern Bering Sea - Donald E. Rogers . Forage Fish Use of Inshore Habitats North of the Alaska Peninsula - Jonathan P. Houghton ................................. Forage Fishes in the Shallow Waters of the North- leut ti an Shelf - Peter Craig ... Population Dynamics of Pacific Herring (Clupea pallasii), Capelin (Mallotus villosus), and Other Coastal Pelagic Fishes in the Eastern Bering Sea - Vidar G. Wespestad The History of Pacific Herring (Clupea pallasii) Fisheries in Alaska - Fritz Funk . Environmental-Dependent Stock-Recruitment Models for Pacific Herring (Clupea pallasii) - Max Stocker. -

Draft Genome of the Korean Smelt Hypomesus Nipponensis and Its Transcriptomic
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437215; this version posted March 28, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 2 3 Draft Genome of the Korean smelt Hypomesus nipponensis and its transcriptomic 4 responses to heat stress in the liver and muscle 5 Biao Xuan1,2, Jongbin Park1,2, Sukjung Choi2, Inhwan You1,2, Bo-Hye Nam3, Eun Soo 6 Noh3, Eun Mi Kim3, Mi-Young Song4, Younhee Shin5, Ji-Hyeon Jeon5,6 and Eun Bae 7 Kim1,2,# 8 1 Department of Applied Animal Science, College of Animal Life Sciences, Kangwon National 9 University, Chuncheon 24341, Kangwon-do, Republic of Korea 10 2 Laboratory of Microbial Genomics and Big Data, College of Animal Life Sciences, Kangwon 11 National University, Chuncheon 24341, Kangwon-do, Republic of Korea 12 3 Biotechnology Research Division, National Institute of Fisheries Science, Busan 46083, Korea 13 4 Inland Fisheries Research Institute, National Institute of Fisheries Science, Gapyeong 12453, 14 Korea 15 5 Research and Development Center, Insilicogen Inc, Yongin 16954, Republic of Korea 16 6 Department of Biological Science, Sungkyunkwan University, Suwon 16419, Korea 17 18 # Corresponding author 19 Mailing address: Department of Applied Animal Science, College of Animal Life Sciences, 20 Kangwon National University, Chuncheon 200-701, Republic of Korea. 21 Tel: +82-33-250-8642 22 Fax: +82-33-259-5574 23 E-mail: [email protected] 24 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437215; this version posted March 28, 2021. -

KLMN Featured Creature Sculpins
National Park Service Featured Creature U.S. Department of the Interior February 2021 Klamath Network Inventory & Monitoring Division Natural Resources Stewardship & Science Sculpins Cottidae General Description Habitat and Distribution Darting low through tide pools or lurking Sculpins occur in both marine and freshwater in stream bottoms, members of the large habitats of North America, Europe, and Asia, fish family, Cottidae, are commonly called with just a few marine species in the southern USFWS/ROGER TABOR sculpins. They also go by “bullhead” or “sea hemisphere. Most abundant in the North Prickly sculpin (Cottus asper) scorpion,” and even some very unflattering Pacific, they tend to frequent shallow water terms, like “double uglies.” You’re not likely and tide pools. In North American coldwa- to catch one on your fishing line, but if you ter streams, they overlap the same habitat as them to keep them oxygenated until they look carefully into ocean tide pools, you trout and salmon, including small headwater hatch a few weeks later into baby fish, known may spot these well camouflaged creatures streams, lakes, and rocky areas of lowland as fry. The fry will be sexually mature in time moving around the bottom. Most of the more rivers. Freshwater sculpin are sometimes the for the next breeding season. than 250–300 known species in this family are only abundant fish species in streams. Inland marine, though some live in freshwater. species found in Pacific Northwest streams Fun Facts include the riffle sculpin (Cottus gulosus), • Some sculpins are able to compress their Generally, sculpins are bottom-dwelling prickly sculpin (Cottus asper), and coastrange skull bones to fit inside small spaces. -
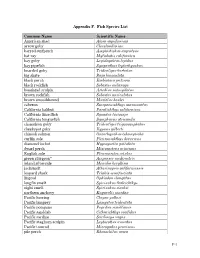
Appendix E: Fish Species List
Appendix F. Fish Species List Common Name Scientific Name American shad Alosa sapidissima arrow goby Clevelandia ios barred surfperch Amphistichus argenteus bat ray Myliobatis californica bay goby Lepidogobius lepidus bay pipefish Syngnathus leptorhynchus bearded goby Tridentiger barbatus big skate Raja binoculata black perch Embiotoca jacksoni black rockfish Sebastes melanops bonehead sculpin Artedius notospilotus brown rockfish Sebastes auriculatus brown smoothhound Mustelus henlei cabezon Scorpaenichthys marmoratus California halibut Paralichthys californicus California lizardfish Synodus lucioceps California tonguefish Symphurus atricauda chameleon goby Tridentiger trigonocephalus cheekspot goby Ilypnus gilberti chinook salmon Oncorhynchus tshawytscha curlfin sole Pleuronichthys decurrens diamond turbot Hypsopsetta guttulata dwarf perch Micrometrus minimus English sole Pleuronectes vetulus green sturgeon* Acipenser medirostris inland silverside Menidia beryllina jacksmelt Atherinopsis californiensis leopard shark Triakis semifasciata lingcod Ophiodon elongatus longfin smelt Spirinchus thaleichthys night smelt Spirinchus starksi northern anchovy Engraulis mordax Pacific herring Clupea pallasi Pacific lamprey Lampetra tridentata Pacific pompano Peprilus simillimus Pacific sanddab Citharichthys sordidus Pacific sardine Sardinops sagax Pacific staghorn sculpin Leptocottus armatus Pacific tomcod Microgadus proximus pile perch Rhacochilus vacca F-1 plainfin midshipman Porichthys notatus rainwater killifish Lucania parva river lamprey Lampetra -

Fishery Management Plan for Groundfish of the Bering Sea and Aleutian Islands Management Area APPENDICES
FMP for Groundfish of the BSAI Management Area Fishery Management Plan for Groundfish of the Bering Sea and Aleutian Islands Management Area APPENDICES Appendix A History of the Fishery Management Plan ...................................................................... A-1 A.1 Amendments to the FMP ......................................................................................................... A-1 Appendix B Geographical Coordinates of Areas Described in the Fishery Management Plan ..... B-1 B.1 Management Area, Subareas, and Districts ............................................................................. B-1 B.2 Closed Areas ............................................................................................................................ B-2 B.3 PSC Limitation Zones ........................................................................................................... B-18 Appendix C Summary of the American Fisheries Act and Subtitle II ............................................. C-1 C.1 Summary of the American Fisheries Act (AFA) Management Measures ............................... C-1 C.2 Summary of Amendments to AFA in the Coast Guard Authorization Act of 2010 ................ C-2 C.3 American Fisheries Act: Subtitle II Bering Sea Pollock Fishery ............................................ C-4 Appendix D Life History Features and Habitat Requirements of Fishery Management Plan SpeciesD-1 D.1 Walleye pollock (Theragra calcogramma) ............................................................................ -

Evolutionary Genomics of a Plastic Life History Trait: Galaxias Maculatus Amphidromous and Resident Populations
EVOLUTIONARY GENOMICS OF A PLASTIC LIFE HISTORY TRAIT: GALAXIAS MACULATUS AMPHIDROMOUS AND RESIDENT POPULATIONS by María Lisette Delgado Aquije Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at Dalhousie University Halifax, Nova Scotia August 2021 Dalhousie University is located in Mi'kma'ki, the ancestral and unceded territory of the Mi'kmaq. We are all Treaty people. © Copyright by María Lisette Delgado Aquije, 2021 I dedicate this work to my parents, María and José, my brothers JR and Eduardo for their unconditional love and support and for always encouraging me to pursue my dreams, and to my grandparents Victoria, Estela, Jesús, and Pepe whose example of perseverance and hard work allowed me to reach this point. ii TABLE OF CONTENTS LIST OF TABLES ............................................................................................................ vii LIST OF FIGURES ........................................................................................................... ix ABSTRACT ...................................................................................................................... xii LIST OF ABBREVIATION USED ................................................................................ xiii ACKNOWLEDGMENTS ................................................................................................ xv CHAPTER 1. INTRODUCTION ....................................................................................... 1 1.1 Galaxias maculatus .................................................................................................. -

Report on the Monitoring of Radionuclides in Fishery Products (March 2011 - January 2015)
Report on the Monitoring of Radionuclides in Fishery Products (March 2011 - January 2015) April 2015 Fisheries Agency of Japan 0 1 Table of Contents Overview…………………………………………………………………………………………………. 8 The Purpose of this Report………………………………………………………………………………9 Part One. Efforts to Guarantee the Safety of Fishery Products………………………………………..11 Chapter 1. Monitoring of Radioactive Materials in Food; Restrictions on Distribution and Other Countermeasures………...…………………………………………………………………11 1-1-1 Standard Limits for Radioactive Materials in Food………………………………………...……11 1-1-2 Methods of Testing for Radioactive Materials………………………………………...…………12 1-1-3 Inspections of Fishery Products for Radioactive Materials…………………………...…………14 1-1-4 Restrictions and Suspensions on Distribution and Shipping ……………………………………..18 1-1-5 Cancellation of Restrictions on Shipping and Distribution………………………………………20 Box 1 Calculation of the Limits for Human Consumption……..………………………………………23 Box 2 Survey of Radiation Dose from Radionuclides in Foods Calculation of the Limits…………….24 Box 3 Examples of Local Government Monitoring Plan………………………………...…………….25 Chapter 2. Results of Radioactive Cesium Inspections for Fishery Products…………………………26 1-2-1 Inspection Results for Nationwide Fishery Products in Japan (in total)…………………………26 1-2-2 Inspection Results for Fukushima Prefecture Fishery Products (all)…………………………….27 1-2-3 Inspection Results for Fishery Products (all) from Outside Fukushima Prefecture……………...30 1-2-4 Trends within Fish Species……………………………………………………………………….32 1-2-5 Inspection Results for Main Target Fish Species of Fishing and Farming by Fiscal Year……….42 1-2-6 Radioactive Material Concentrations within Fish within 20 km of the Fukushima Daiichi NPS.46 Box 4 Fukushima Fishing Trials………………………………...……………………………………...47 1-2-7 Screening Test by Prefectural and Municipal Governments……………………………………..48 Chapter 3. Inspection for Radionuclides Other Than Radioactive Cesium……………………………49 1-3-1 Inspections for Radioactive Strontium etc. -

Historical Fish Specimens Collected from the Tohoku District by the Saito Ho-On Kai Museum of Natural History
Bull. Natl. Mus. Nat. Sci., Ser. A, 35(1), pp. 9–54, March 22, 2009 Historical Fish Specimens Collected from the Tohoku District by the Saito Ho-on Kai Museum of Natural History Keiichi Matsuura1, Gento Shinohara2 and Masanori Nakae1 1 Collection Center, National Museum of Nature and Science, 3–23–1 Hyakunin-cho, Shinjuku-ku, Tokyo, 169–0073 Japan E-mail: [email protected]; [email protected] 2 Department of Zoology, National Museum of Nature and Science, 3–23–1 Hyakunin-cho, Shinjuku-ku, Tokyo, 169–0073 Japan E-mail: [email protected] Abstract The fish collection of the Saito Ho-on Kai Museum of Natural History was transferred to the National Museum of Nature and Science, Tokyo in February 2006. Ninety percent of the fish collection contains specimens collected from the Tohoku District during the period from 1930 to 1933 when natural environments of Japan were in good condition for various groups of fishes. The fish specimens from the Tohoku District were classified into 361 species/subspecies of 273 genera belonging to 131 families of 31 orders. A list of the species is shown with remarks on distribution. Key words: Fish specimens, Saito Ho-on Kai Museum, Tohoku District, inventory. stead of natural sicence. The museum has tried to Introduction keep its activity at the level before the war, but it The Saito Ho-on Kai Museum was established failed to do so because of financial difficulties. In in November 1933 in Sendai City, Miyagi Pre- 2005, the Saito Ho-on Kai Museum of Natural fecture, Japan. -

The Intramandibular Joint in Girella: a Mechanism for Increased Force Production?
JOURNAL OF MORPHOLOGY 271:271–279 (2010) The Intramandibular Joint in Girella: A Mechanism for Increased Force Production? Lara A. Ferry-Graham1,3* and Nicolai Konow2 1California State University, Moss Landing Marine Labs, Moss Landing, California 95039 2Brown University, Ecology and Evolutionary Biology, Providence, Box G-B204, Rhode Island 02912 3CEAZA, Centro de Estudios Avanzados de Zonas Aridas (Center for Advanced Studies in Arid Zones), Universidad Cato´lica del Norte. Box 599, Benavente 980, La Serena, Chile ABSTRACT Intramandibular joints (IMJ) are novel Bellwood, 2002). This is likely due to the forces articulations between bony elements of the lower jaw required for obtaining food items many of which are that have evolved independently in multiple fish line- either tough or firmly attached to the substrate ages and are typically associated with biting herbivory. (Alfaro et al., 2001). In several reef fish lineages, This novel joint is hypothesized to function by augment- aspects of the feeding apparatus have been radically ing oral jaw expansion during mouth opening, which would increase contact between the tooth-bearing area altered for force production, either via hypertro- of the jaws and algal substratum during feeding, result- phied or via structurally altered musculature (Friel ing in more effective food removal from the substrate. and Wainwright, 1997), modified muscle attach- Currently, it is not understood if increased flexibility in ments (Vial and Ojeda, 1990; Konow and Bellwood, a double-jointed mandible also results in increased force 2005), or increased suturing and/or reinforcement generation during herbivorous biting and/or scraping. of bony elements and dentition (Tedman, 1980; Therefore, we selected the herbivore Girella laevifrons Streelman et al., 2002; Bellwood et al., 2003). -

Humboldt Bay Fishes
Humboldt Bay Fishes ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> ·´¯`·._.·´¯`·.. ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> Acknowledgements The Humboldt Bay Harbor District would like to offer our sincere thanks and appreciation to the authors and photographers who have allowed us to use their work in this report. Photography and Illustrations We would like to thank the photographers and illustrators who have so graciously donated the use of their images for this publication. Andrey Dolgor Dan Gotshall Polar Research Institute of Marine Sea Challengers, Inc. Fisheries And Oceanography [email protected] [email protected] Michael Lanboeuf Milton Love [email protected] Marine Science Institute [email protected] Stephen Metherell Jacques Moreau [email protected] [email protected] Bernd Ueberschaer Clinton Bauder [email protected] [email protected] Fish descriptions contained in this report are from: Froese, R. and Pauly, D. Editors. 2003 FishBase. Worldwide Web electronic publication. http://www.fishbase.org/ 13 August 2003 Photographer Fish Photographer Bauder, Clinton wolf-eel Gotshall, Daniel W scalyhead sculpin Bauder, Clinton blackeye goby Gotshall, Daniel W speckled sanddab Bauder, Clinton spotted cusk-eel Gotshall, Daniel W. bocaccio Bauder, Clinton tube-snout Gotshall, Daniel W. brown rockfish Gotshall, Daniel W. yellowtail rockfish Flescher, Don american shad Gotshall, Daniel W. dover sole Flescher, Don stripped bass Gotshall, Daniel W. pacific sanddab Gotshall, Daniel W. kelp greenling Garcia-Franco, Mauricio louvar