Forage Fishes of the Southeastern Bering Sea Conference Proceedings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bay Shore Power Plant Cooling Water Intake Structure Information and I&E Sampling Data
BAY SHORE POWER PLANT COOLING WATER INTAKE STRUCTURE INFORMATION AND I&E SAMPLING DATA Kinectrics Report: 112026-005-RA-0002-R00 January, 2008 Darlene Ager, Ph.D., David Marttila, Eng, Paul Patrick, Ph.D. Environmental and Aquatic Management Services PRIVATE INFORMATION Contents of this report shall not be disclosed without the consent of the Customer. Kinectrics has prepared this report in accordance with and subject to the contract Terms and Conditions between Kinectrics and FirstEnergy, dated October 7, 2004 © Kinectrics North America Inc., 2008 Kinectrics North America Inc., 800 Kipling Avenue Toronto, Ontario, Canada M8Z 6C4 BAY SHORE POWER PLANT COOLING WATER INTAKE STRUCTURE Kinectrics Report: 112026-005-RA-0002-R00 January, 2008 Darlene Ager, Ph.D., David Marttila, Eng. Paul Patrick, Ph.D. Environmental and Aquatic Management Services EXECUTIVE SUMMARY Section 316(b) of the Clean Water Act (CWA) requires that cooling water intake structures reflect the best technology available for minimizing adverse environmental impact to aquatic organisms that are impinged (being pinned against screens or outer part of a cooling water intake structure) or entrained (being drawn into and through cooling water systems). Phase II of the 316(b) rule for existing electric generating plants was designed to reduce impingement mortality by 80-95% and, if applicable, entrainment by 60-90%. In January 2007, the Second U.S. Circuit Court of Appeals remanded several provisions of the Phase II rule on various grounds. The provisions remanded included: • EPA’s determination of the Best Technology Available under Section 316(b); • The rule’s performance standard ranges; • The cost-cost and cost-benefit compliance alternatives; • The Technology Installation and Operation Plan provision; • The restoration provisions; and • The “independent supplier” provision. -

Fishery Management Plan for Arctic Grayling Sport Fisheries Along the Nome Road System, 2001–2004
Fishery Management Report No. 02-03 Fishery Management Plan for Arctic Grayling Sport Fisheries along the Nome Road System, 2001–2004 by Fred DeCicco April 2002 Alaska Department of Fish and Game Division of Sport Fish Symbols and Abbreviations The following symbols and abbreviations, and others approved for the Système International d'Unités (SI), are used in Division of Sport Fish Fishery Manuscripts, Fishery Data Series Reports, Fishery Management Reports, and Special Publications without definition. Weights and measures (metric) General Mathematics, statistics, fisheries centimeter cm All commonly accepted e.g., Mr., Mrs., alternate hypothesis HA deciliter dL abbreviations. a.m., p.m., etc. base of natural e gram g All commonly accepted e.g., Dr., Ph.D., logarithm hectare ha professional titles. R.N., etc. catch per unit effort CPUE kilogram kg and & coefficient of variation CV at @ 2 kilometer km common test statistics F, t, , etc. liter L Compass directions: confidence interval C.I. meter m east E correlation coefficient R (multiple) north N metric ton mt correlation coefficient r (simple) milliliter ml south S covariance cov millimeter mm west W degree (angular or ° Copyright temperature) Weights and measures (English) Corporate suffixes: degrees of freedom df cubic feet per second ft3/s Company Co. divided by ÷ or / (in foot ft Corporation Corp. equations) gallon gal Incorporated Inc. equals = inch in Limited Ltd. expected value E mile mi et alii (and other et al. fork length FL ounce oz people) greater than > O pound lb et cetera (and so forth) etc. greater than or equal to quart qt exempli gratia (for e.g., harvest per unit effort HPUE example) yard yd less than < id est (that is) i.e., ? less than or equal to latitude or longitude lat. -

Pamphlet to Accompany Scientific Investigations Map 3131
Bedrock Geologic Map of the Seward Peninsula, Alaska, and Accompanying Conodont Data By Alison B. Till, Julie A. Dumoulin, Melanie B. Werdon, and Heather A. Bleick Pamphlet to accompany Scientific Investigations Map 3131 View of Salmon Lake and the eastern Kigluaik Mountains, central Seward Peninsula 2011 U.S. Department of the Interior U.S. Geological Survey Contents Introduction ....................................................................................................................................................1 Sources of data ....................................................................................................................................1 Components of the map and accompanying materials .................................................................1 Geologic Summary ........................................................................................................................................1 Major geologic components ..............................................................................................................1 York terrane ..................................................................................................................................2 Grantley Harbor Fault Zone and contact between the York terrane and the Nome Complex ..........................................................................................................................3 Nome Complex ............................................................................................................................3 -

Conservation and Ecology of Marine Forage Fishes— Proceedings of a Research Symposium, September 2012
Conservation and Ecology of Marine Forage Fishes— Proceedings of a Research Symposium, September 2012 Open-File Report 2013–1035 U.S. Department of the Interior U.S. Geological Survey Cover: Upper Left: Herring spawn, BC coast – Milton Love, “Certainly More Than You Want to Know About the Fishes of the Pacific Coast,” reproduced with permission. Left Center: Tufted Puffin and sand lance (Smith Island, Puget Sound) – Joseph Gaydos, SeaDoc Society. Right Center: Symposium attendants – Tami Pokorny, Jefferson County Water Resources. Upper Right: Buried sand lance – Milton Love, “Certainly More Than You Want to Know About the Fishes of the Pacific Coast,” reproduced with permission. Background: Pacific sardine, CA coast – Milton Love, “Certainly More Than You Want to Know About the Fishes of the Pacific Coast,” reproduced with permission. Conservation and Ecology of Marine Forage Fishes— Proceedings of a Research Symposium, September 2012 Edited by Theresa Liedtke, U.S. Geological Survey; Caroline Gibson, Northwest Straits Commission; Dayv Lowry, Washington State Department of Fish and Wildlife; and Duane Fagergren, Puget Sound Partnership Open-File Report 2013–1035 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2013 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov or call 1–888–ASK–USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Suggested citation: Liedtke, Theresa, Gibson, Caroline, Lowry, Dayv, and Fagergren, Duane, eds., 2013, Conservation and Ecology of Marine Forage Fishes—Proceedings of a Research Symposium, September 2012: U.S. -

Atlantic "Pelagic" Fish Underwater World
QL DFO - Library / MPO - Bibliothèque 626 U5313 no.3 12064521 c.2 - 1 Atlantic "Pelagic" Fish Underwater World Fish that range the open sea are Pelagic species are generally very Atlantic known as " pelagic" species, to dif streamlined. They are blue or blue ferentiate them from "groundfish" gray over their backs and silvery "Pelagic" Fish which feed and dwell near the bot white underneath - a form of tom . Feeding mainly in surface or camouflage when in the open sea. middle depth waters, pelagic fish They are caught bath in inshore travel mostly in large schools, tu. n and offshore waters, principally with ing and manoeuvring in close forma mid-water trawls, purse seines, gill tion with split-second timing in their nets, traps and weirs. quest for plankton and other small species. Best known of the pelagic popula tions of Canada's Atlantic coast are herring, but others in order of economic importance include sal mon, mackerel , swordfish, bluefin tuna, eels, smelt, gaspereau and capelin. Sorne pelagic fish, notably salmon and gaspereau, migrate from freshwater to the sea and back again for spawning. Eels migrate in the opposite direction, spawning in sait water but entering freshwater to feed . Underwater World Herring comprise more than one Herring are processed and mar Atlantic Herring keted in various forms. About half of (Ctupea harengus) fifth of Atlantic Canada's annual fisheries catch. They are found all the catch is marketed fresh or as along the northwest Atlantic coast frozen whole dressed fish and fillets, from Cape Hatteras to Hudson one-quarter is cured , including Strait. -

Vii Fishery-At-A-Glance Night Smelt Scientific Name: Spirinchus Starksi Range
Fishery-at-a-Glance Night Smelt Scientific Name: Spirinchus starksi Range: Night Smelt are distributed coast-wide from southeast Alaska to Point Arguello, Santa Barbara County. Habitat: Night Smelt occur in the surf and in depths from the surface to approximately 400 feet (122 meters). Size (length and weight): Night Smelt measure less than 6 inches total length (140 millimeters) weighing to 11 grams. Males are slightly longer and heavier than females. Life span: Night Smelt are short lived and believed to reach a maximum of 2 to 3 years. Reproduction: Spawning occurs in the surf along open coast coarse sand beaches from January to September. Eggs are fertilized in the wash of the surf, adhere to sand grains, and sink. Hatching occurs in approximately 2 weeks. Prey: Night Smelt feed on small crustaceans—primarily gammarid amphipods and mysid shrimp. Predators: Night Smelt provide forage for a wide range of predators, including Striped Bass, Redtail Surfperch, salmon, Harbor Seals, California Sea Lions, terns, gulls, and cormorants. Fishery: Commercial and recreational fisheries are shore-based. Area fished: Historically, fishing occurred from Moss Landing, Monterey County to the Oregon border. Currently, fishing occurs from San Mateo County to Del Norte County. Fishing season: Fishing occurs during the spawning season—January to September. Fishing gear: Fishermen fish from shore using A-frame dip nets. Market(s): Landed fish are sold for human consumption and aquarium food. Current stock status: No formal stock assessments exist for Night Smelt. Although catch rates have increased on average since the early 2000s, it is undetermined if this increase in the index is due to increased abundance or changes in fishermen behavior. -

Forage Fish Management Plan
Oregon Forage Fish Management Plan November 19, 2016 Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-4741 http://www.dfw.state.or.us/MRP/ Oregon Department of Fish & Wildlife 1 Table of Contents Executive Summary ....................................................................................................................................... 4 Introduction .................................................................................................................................................. 6 Purpose and Need ..................................................................................................................................... 6 Federal action to protect Forage Fish (2016)............................................................................................ 7 The Oregon Marine Fisheries Management Plan Framework .................................................................. 7 Relationship to Other State Policies ......................................................................................................... 7 Public Process Developing this Plan .......................................................................................................... 8 How this Document is Organized .............................................................................................................. 8 A. Resource Analysis .................................................................................................................................... -

Rainbow Smelt Spawning Monitoring
PROGRESS REPORT State: NEW HAMPSHIRE Grant: F-61-R-22/F19AF00061 Grant Title: NEW HAMPSHIRE’S MARINE FISHERIES INVESTIGATIONS Project I: DIADROMOUS FISH INVESTIGATIONS Job 2: MONITORING OF RAINBOW SMELT SPAWNING ACTIVITY Objective: To annually monitor the Rainbow Smelt Osmerus mordax resource using fishery independent techniques during their spawning run in the Great Bay Estuary. Period Covered: January 1, 2019 - December 31, 2019 ABSTRACT In 2019, a total of 844 Rainbow Smelt Osmerus mordax (349 in Oyster River, 405 in Winnicut River, and 90 in Squamscott River) were caught in fyke nets. The CPUE in 2019 was highest in the Oyster River with 23.79 smelt per day, whereas the Winnicut River (8.46 smelt per day) and Squamscott River (5.54 smelt per day) were lower. A male-skewed sex ratio was observed at all rivers, a likely result of differences in spawning behavior between sexes. The age distribution of captured Rainbow Smelt, weighted by total catch was highest for age-2 fish, followed by age-1, age-3, and age-4 fish. Most water quality measurements (temperature, dissolved oxygen, specific conductivity, and pH) were within or near acceptable ranges for smelt spawning and egg incubation and development in 2019; however, turbidity was above the threshold in the Oyster River for most days monitored. INTRODUCTION Rainbow Smelt Osmerus mordax are small anadromous fish that live in nearshore coastal waters and spawn in the spring in tidal rivers immediately above the head of tide in freshwater (Kendall 1926; Murawski et al. 1980; Buckley 1989). Anadromous smelt serve as important prey for commercial and recreational culturally valuable species, such as Atlantic Cod Gadus morhua, Atlantic Salmon Salmo salar, and Striped Bass Morone saxatilis (Clayton et al. -
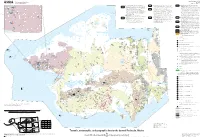
Digital Data for the Preliminary Bedrock Geologic Map of The
Open-File Report 2009-1254 U.S. Department of the Interior Sheet 2 of 2 U.S. Geological Survey Pamphlet accompanies map '02°761 '01°761 '00°761 LIST OF METAMORPHIC-TECTONIC ELEMENTS HIGH GRADE METAMORPHIC AND ASSOCIATED O<t Qs Ol Nome Complex, west-central – Weakly foliated metasedimentary O<l O<l IGNEOUS ROCKS – Amphibolite and granulite-facies O<l O<l Ol Ols Ols YORK TERRANE – Late Proterozoic (?) and Paleozoic sedimentary and unfoliated metaigneous rocks that retain relict primary features; and metasedimentary rocks and minor Late Cretaceous tin-bearing mineral assemblages in mafic rocks formed at pumpellyite-actinolite, metamorphic rocks and associated Cretaceous plutons; penetratively Qs greenschist, and blueschist facies (one locality) deformed metasedimentary and metaigneous schist and gneiss with Oal granites; dominantly carbonate and siliciclastic lithologies, in which Oal primary features are generally retained; fine-grained Nome Complex, eastern – Penetratively deformed and complex metamorphic histories; aluminum-rich lithologies show Ol early development of kyanite-stable mineral assemblages succeeded Ol 17 metasedimentary rocks are weakly foliated. Metamorphic and recrystallized schists with ductile fabrics; protolith packages and Ol Ols 165°00' by sillimanite-stable, lower-pressure assemblages. Lithologies rich in O<p Ols thermal history variable from unit to unit, and generally lower grade metamorphic fabrics identical to Nome Complex in central Seward Oal Qs Peninsula; mineral assemblages in most of the area are characteristic iron and aluminum retain early, relatively high pressure Ktg than Nome Complex. Tin granites intruded in shallow crustal Ols Ol aluminosilicate plus orthoamphibole assemblages (>5kb) that are Cape Espenberg settings. The generally brittle shallow and steeply-dipping structures of greenschist facies, but slightly higher grade assemblages occur in Ol the vicinity of Kiwalik Mountain. -

The PAYSTREAK Volume 12, No
The PAYSTREAK Volume 12, No. 2, Fall 2010 The Newsletter of the Alaska Mining Hall of Fame Foundation (AMHF) In this issue: AMHF New Inductees Page 1 Induction Ceremony Program Page 2 Introduction and Acknowledgements Page 3 Previous AMHF Inductees Pages 4 - 10 New Inductee Biographies Pages 10 - 23 Distinguished Alaskans Aid Foundation Page 24 Alaska Mining Hall of Fame Directors and Officers Page 24 Alaska Mining Hall of Fame Foundation New Inductees AMHF Honors Pioneers Important to the Seward Peninsula Gold Dredging Industry Nicholas B. and Evinda S. Tweet: It is difficult to name any couple in Alaska mining history that has had more longevity and perseverance than Nicholas B. and Evinda S. Tweet. In marriage, they formed a team that created a remarkably stable, family-owned firm, N.B. Tweet and Sons, which has mined gold in Alaska for 110 years. Nicholas and Evinda mined and lived in several placer mining camps, worked graphite claims, operated gold dredges, and inspired their descendants to continue the placer mining lifestyle. Both Evinda and Nick died at the family mining camp, near Taylor, north of Nome. In 2010, N.B. Tweet and Sons operated the only bucketline stacker gold dredge in North America. Carl S. (left) and Walter A. (right) Glavinovich: Two Croatian (Yugoslavian)-born brothers who, collectively, devoted more than one-hundred years of their lives to the prospecting, deciphering, drilling, thawing, and dredging of the Nome, Alaska, placer gold fields. Most of these years were in the service of one company, the U.S. Smelting, Refining, and Mining Company (USSR&M) or its direct affiliate, the Hammon Consolidated Gold Fields. -

Humboldt Bay Fishes
Humboldt Bay Fishes ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> ·´¯`·._.·´¯`·.. ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> Acknowledgements The Humboldt Bay Harbor District would like to offer our sincere thanks and appreciation to the authors and photographers who have allowed us to use their work in this report. Photography and Illustrations We would like to thank the photographers and illustrators who have so graciously donated the use of their images for this publication. Andrey Dolgor Dan Gotshall Polar Research Institute of Marine Sea Challengers, Inc. Fisheries And Oceanography [email protected] [email protected] Michael Lanboeuf Milton Love [email protected] Marine Science Institute [email protected] Stephen Metherell Jacques Moreau [email protected] [email protected] Bernd Ueberschaer Clinton Bauder [email protected] [email protected] Fish descriptions contained in this report are from: Froese, R. and Pauly, D. Editors. 2003 FishBase. Worldwide Web electronic publication. http://www.fishbase.org/ 13 August 2003 Photographer Fish Photographer Bauder, Clinton wolf-eel Gotshall, Daniel W scalyhead sculpin Bauder, Clinton blackeye goby Gotshall, Daniel W speckled sanddab Bauder, Clinton spotted cusk-eel Gotshall, Daniel W. bocaccio Bauder, Clinton tube-snout Gotshall, Daniel W. brown rockfish Gotshall, Daniel W. yellowtail rockfish Flescher, Don american shad Gotshall, Daniel W. dover sole Flescher, Don stripped bass Gotshall, Daniel W. pacific sanddab Gotshall, Daniel W. kelp greenling Garcia-Franco, Mauricio louvar -

Climate-Ocean Effects on AYK Chinook Salmon
SAFS-UW-1003 2010 Arctic Yukon Kuskokwim (AYK) Sustainable Salmon Initiative Project Final Product1 Climate-Ocean Effects on AYK Chinook Salmon Chukchi Sea by Katherine W. Myers2, Robert V. Walker2, Nancy D. Davis2, Janet L. Armstrong2, Wyatt J. Fournier2, Nathan J. Mantua2, and Julie Raymond-Yakoubian 3 2High Seas Salmon Research Program, School of Aquatic & Fishery Sciences (SAFS), University of Washington (UW), Box 355020, Seattle, WA 98195-5020, USA 3Kawerak, Inc., PO Box 948, Nome, AK 99762, USA November 2010 1Final products of AYK Sustainable Salmon Initiative (SSI) research are made available to the Initiatives partners and the public in the interest of rapid dissemination of information that may be useful in salmon management, research, or administration. Sponsorship of the project by the AYK SSI does not necessarily imply that the findings or conclusions are endorsed by the AYK SSI. ABSTRACT A high-priority research issue identified by the Arctic-Yukon-Kuskokwim (AYK) Sustainable Salmon Initiative (SSI) is to determine whether the ocean environment is a more important cause of variation in the abundance of AYK Pacific salmon (Oncorhynchus spp.) populations than marine fishing mortality. At the outset of this project, however, data on ocean life history of AYK salmon were too limited to test hypotheses about the effects of environmental conditions versus fishing on marine survival. Our goal was to identify and evaluate life history patterns of use of marine resources (habitat and food) by Chinook salmon (O. tshawytscha) and to explore how these patterns are affected by climate-ocean conditions, including documentation of local traditional knowledge (LTK) of this high-priority issue.