A Dissertation Entitled Evolution, Systematics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review and Updated Checklist of Freshwater Fishes of Iran: Taxonomy, Distribution and Conservation Status
Iran. J. Ichthyol. (March 2017), 4(Suppl. 1): 1–114 Received: October 18, 2016 © 2017 Iranian Society of Ichthyology Accepted: February 30, 2017 P-ISSN: 2383-1561; E-ISSN: 2383-0964 doi: 10.7508/iji.2017 http://www.ijichthyol.org Review and updated checklist of freshwater fishes of Iran: Taxonomy, distribution and conservation status Hamid Reza ESMAEILI1*, Hamidreza MEHRABAN1, Keivan ABBASI2, Yazdan KEIVANY3, Brian W. COAD4 1Ichthyology and Molecular Systematics Research Laboratory, Zoology Section, Department of Biology, College of Sciences, Shiraz University, Shiraz, Iran 2Inland Waters Aquaculture Research Center. Iranian Fisheries Sciences Research Institute. Agricultural Research, Education and Extension Organization, Bandar Anzali, Iran 3Department of Natural Resources (Fisheries Division), Isfahan University of Technology, Isfahan 84156-83111, Iran 4Canadian Museum of Nature, Ottawa, Ontario, K1P 6P4 Canada *Email: [email protected] Abstract: This checklist aims to reviews and summarize the results of the systematic and zoogeographical research on the Iranian inland ichthyofauna that has been carried out for more than 200 years. Since the work of J.J. Heckel (1846-1849), the number of valid species has increased significantly and the systematic status of many of the species has changed, and reorganization and updating of the published information has become essential. Here we take the opportunity to provide a new and updated checklist of freshwater fishes of Iran based on literature and taxon occurrence data obtained from natural history and new fish collections. This article lists 288 species in 107 genera, 28 families, 22 orders and 3 classes reported from different Iranian basins. However, presence of 23 reported species in Iranian waters needs confirmation by specimens. -
Appendix E: Fish Species List
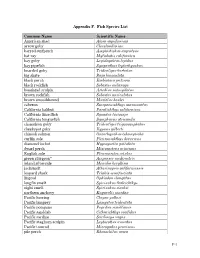
Appendix F. Fish Species List Common Name Scientific Name American shad Alosa sapidissima arrow goby Clevelandia ios barred surfperch Amphistichus argenteus bat ray Myliobatis californica bay goby Lepidogobius lepidus bay pipefish Syngnathus leptorhynchus bearded goby Tridentiger barbatus big skate Raja binoculata black perch Embiotoca jacksoni black rockfish Sebastes melanops bonehead sculpin Artedius notospilotus brown rockfish Sebastes auriculatus brown smoothhound Mustelus henlei cabezon Scorpaenichthys marmoratus California halibut Paralichthys californicus California lizardfish Synodus lucioceps California tonguefish Symphurus atricauda chameleon goby Tridentiger trigonocephalus cheekspot goby Ilypnus gilberti chinook salmon Oncorhynchus tshawytscha curlfin sole Pleuronichthys decurrens diamond turbot Hypsopsetta guttulata dwarf perch Micrometrus minimus English sole Pleuronectes vetulus green sturgeon* Acipenser medirostris inland silverside Menidia beryllina jacksmelt Atherinopsis californiensis leopard shark Triakis semifasciata lingcod Ophiodon elongatus longfin smelt Spirinchus thaleichthys night smelt Spirinchus starksi northern anchovy Engraulis mordax Pacific herring Clupea pallasi Pacific lamprey Lampetra tridentata Pacific pompano Peprilus simillimus Pacific sanddab Citharichthys sordidus Pacific sardine Sardinops sagax Pacific staghorn sculpin Leptocottus armatus Pacific tomcod Microgadus proximus pile perch Rhacochilus vacca F-1 plainfin midshipman Porichthys notatus rainwater killifish Lucania parva river lamprey Lampetra -

Arrival of Round Goby Neogobius Melanostomus (Pallas, 1814) and Bighead Goby Ponticola Kessleri (Günther, 1861) in the High Rhine (Switzerland)
BioInvasions Records (2013) Volume 2, Issue 1: 79–83 Open Access doi: http://dx.doi.org/10.3391/bir.2013.2.1.14 © 2013 The Author(s). Journal compilation © 2013 REABIC Short Communication Arrival of round goby Neogobius melanostomus (Pallas, 1814) and bighead goby Ponticola kessleri (Günther, 1861) in the High Rhine (Switzerland) Irene Kalchhauser*, Peter Mutzner, Philipp E. Hirsch and Patricia Burkhardt-Holm Program Man-Society-Environment, Dept. of Environmental Sciences, University of Basel, Vesalgasse 1, 4051 Basel, Switzerland E-mail: [email protected] (IK), [email protected] (PM), [email protected] (PEH), [email protected] (PBH) *Corresponding author Received: 20 July 2012 / Accepted: 31 October 2012 / Published online: 22 November 2012 Handling editor: Vadim Panov Abstract A number of Ponto-Caspian gobiid species are currently invading European coasts and freshwaters. They do not only present a nuisance to fishermen, but evidence suggests that they compete with native benthic fishes and may contribute to changes in ecosystem function. This paper reports the presence of round goby Neogobius melanostomus individuals and an established population of bighead goby Ponticola kessleri in the High Rhine. Key words: gobiidae; non-native; alien; invasion; High Rhine; Switzerland 2001) was predicted to promote westward Introduction migration of fish species (Balon et al. 1986), including Ponto-Caspian gobiids (Proterorhinus Several goby species from the Caspian and Black marmoratus). In the meantime, five of six gobiid Sea are currently spreading in European rivers. species predicted to invade the Rhine (Freyhof Ponticola kessleri (Günther, 1861; Neilson and 2003) have indeed arrived. Their dispersal Stepien 2009), Neogobius melanostomus (Pallas, appears to be facilitated by shipping, as round 1814), Proterorhinus marmoratus (Pallas, 1814), goby dispersal has followed shipping routes Neogobius fluviatilis (Pallas, 1814), and Babka (Brown and Stepien 2009; LaRue et al. -

Caspian Goby (Neogobius Caspius) Ecological Risk Screening Summary
Caspian Goby (Neogobius caspius) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, March 2012 Revised, August 2018 Web Version, 8/2/2019 Photo: K. Abbasi. Licensed under CC BY-SA 3.0. Available: http://eol.org/pages/207518/overview. (August 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018): “Europe and Asia: Caspian Sea.” From Eschmeyer et al. (2018): “Distribution: Caspian Sea basin, Eurasia.” Status in the United States There are no known occurrences in the United States. It does not appear that this species is in the trade in the United States based on a search of the literature and online aquarium retailers. Means of Introductions in the United States There are no known occurrences in the United States. 1 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2018): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Actinopterygii Class Teleostei Superorder Acanthopterygii Order Perciformes Suborder Gobioidei Family Gobiidae Genus Neogobius Species Neogobius caspius (Eichwald, 1831)” From Eschmeyer et al. (2018): “Current status: Valid as Neogobius caspius (Eichwald 1831). Gobiidae: Gobiinae.” Size, Weight, and Age Range From Froese and Pauly (2018): “Max length : 34.5 cm TL male/unsexed; [Berg 1965]” Environment From Froese and Pauly (2018): “Brackish; demersal.” Climate/Range From Froese and Pauly (2018): “Temperate” Distribution Outside the United States Native From Froese and Pauly (2018): “Europe and Asia: Caspian Sea.” 2 From Eschmeyer et al. (2018): “Distribution: Caspian Sea basin, Eurasia.” Introduced No known introductions. Means of Introduction Outside the United States No known introductions. -

Report on the Monitoring of Radionuclides in Fishery Products (March 2011 - January 2015)
Report on the Monitoring of Radionuclides in Fishery Products (March 2011 - January 2015) April 2015 Fisheries Agency of Japan 0 1 Table of Contents Overview…………………………………………………………………………………………………. 8 The Purpose of this Report………………………………………………………………………………9 Part One. Efforts to Guarantee the Safety of Fishery Products………………………………………..11 Chapter 1. Monitoring of Radioactive Materials in Food; Restrictions on Distribution and Other Countermeasures………...…………………………………………………………………11 1-1-1 Standard Limits for Radioactive Materials in Food………………………………………...……11 1-1-2 Methods of Testing for Radioactive Materials………………………………………...…………12 1-1-3 Inspections of Fishery Products for Radioactive Materials…………………………...…………14 1-1-4 Restrictions and Suspensions on Distribution and Shipping ……………………………………..18 1-1-5 Cancellation of Restrictions on Shipping and Distribution………………………………………20 Box 1 Calculation of the Limits for Human Consumption……..………………………………………23 Box 2 Survey of Radiation Dose from Radionuclides in Foods Calculation of the Limits…………….24 Box 3 Examples of Local Government Monitoring Plan………………………………...…………….25 Chapter 2. Results of Radioactive Cesium Inspections for Fishery Products…………………………26 1-2-1 Inspection Results for Nationwide Fishery Products in Japan (in total)…………………………26 1-2-2 Inspection Results for Fukushima Prefecture Fishery Products (all)…………………………….27 1-2-3 Inspection Results for Fishery Products (all) from Outside Fukushima Prefecture……………...30 1-2-4 Trends within Fish Species……………………………………………………………………….32 1-2-5 Inspection Results for Main Target Fish Species of Fishing and Farming by Fiscal Year……….42 1-2-6 Radioactive Material Concentrations within Fish within 20 km of the Fukushima Daiichi NPS.46 Box 4 Fukushima Fishing Trials………………………………...……………………………………...47 1-2-7 Screening Test by Prefectural and Municipal Governments……………………………………..48 Chapter 3. Inspection for Radionuclides Other Than Radioactive Cesium……………………………49 1-3-1 Inspections for Radioactive Strontium etc. -

Andhra Pradesh
PROFILES OF SELECTED NATIONAL PARKS AND SANCTUARIES OF INDIA JULY 2002 EDITED BY SHEKHAR SINGH ARPAN SHARMA INDIAN INSTITUTE OF PUBLIC ADMINISTRATION NEW DELHI CONTENTS STATE NAME OF THE PA ANDAMAN AND NICOBAR CAMPBELL BAY NATIONAL PARK ISLANDS GALATHEA NATIONAL PARK MOUNT HARRIET NATIONAL PARK NORTH BUTTON ISLAND NATIONAL PARK MIDDLE BUTTON ISLAND NATIONAL PARK SOUTH BUTTON ISLAND NATIONAL PARK RANI JHANSI MARINE NATIONAL PARK WANDOOR MARINE NATIONAL PARK CUTHBERT BAY WILDLIFE SANCTUARY GALATHEA BAY WILDLIFE SANCTUARY INGLIS OR EAST ISLAND SANCTUARY INTERVIEW ISLAND SANCTUARY LOHABARRACK OR SALTWATER CROCODILE SANCTUARY ANDHRA PRADESH ETURUNAGARAM SANCTUARY KAWAL WILDLIFE SANCTUARY KINNERSANI SANCTUARY NAGARJUNASAGAR-SRISAILAM TIGER RESERVE PAKHAL SANCTUARY PAPIKONDA SANCTUARY PRANHITA WILDLIFE SANCTUARY ASSAM MANAS NATIONAL PARK GUJARAT BANSDA NATIONAL PARK PURNA WILDLIFE SANCTUARY HARYANA NAHAR SANCTUARY KALESAR SANCTUARY CHHICHHILA LAKE SANCTUARY ABUBSHEHAR SANCTUARY BIR BARA VAN JIND SANCTUARY BIR SHIKARGAH SANCTUARY HIMACHAL PRADESH PONG LAKE SANCTUARY RUPI BHABA SANCTUARY SANGLA SANCTUARY KERALA SILENT VALLEY NATIONAL PARK ARALAM SANCTUARY CHIMMONY SANCTUARY PARAMBIKULAM SANCTUARY PEECHI VAZHANI SANCTUARY THATTEKAD BIRD SANCTUARY WAYANAD WILDLIFE SANCTUARY MEGHALAYA BALPAKARAM NATIONAL PARK SIJU WILDLIFE SANCTUARY NOKREK NATIONAL PARK NONGKHYLLEM WILDLIFE SANCTUARY MIZORAM MURLEN NATIONAL PARK PHAWNGPUI (BLUE MOUNTAIN) NATIONAL 2 PARK DAMPA WILDLIFE SANCTUARY KHAWNGLUNG WILDLIFE SANCTUARY LENGTENG WILDLIFE SANCTUARY NGENGPUI WILDLIFE -

TNP SOK 2011 Internet
GARDEN ROUTE NATIONAL PARK : THE TSITSIKAMMA SANP ARKS SECTION STATE OF KNOWLEDGE Contributors: N. Hanekom 1, R.M. Randall 1, D. Bower, A. Riley 2 and N. Kruger 1 1 SANParks Scientific Services, Garden Route (Rondevlei Office), PO Box 176, Sedgefield, 6573 2 Knysna National Lakes Area, P.O. Box 314, Knysna, 6570 Most recent update: 10 May 2012 Disclaimer This report has been produced by SANParks to summarise information available on a specific conservation area. Production of the report, in either hard copy or electronic format, does not signify that: the referenced information necessarily reflect the views and policies of SANParks; the referenced information is either correct or accurate; SANParks retains copies of the referenced documents; SANParks will provide second parties with copies of the referenced documents. This standpoint has the premise that (i) reproduction of copywrited material is illegal, (ii) copying of unpublished reports and data produced by an external scientist without the author’s permission is unethical, and (iii) dissemination of unreviewed data or draft documentation is potentially misleading and hence illogical. This report should be cited as: Hanekom N., Randall R.M., Bower, D., Riley, A. & Kruger, N. 2012. Garden Route National Park: The Tsitsikamma Section – State of Knowledge. South African National Parks. TABLE OF CONTENTS 1. INTRODUCTION ...............................................................................................................2 2. ACCOUNT OF AREA........................................................................................................2 -

The Round Goby (Neogobius Melanostomus):A Review of European and North American Literature
ILLINOI S UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN PRODUCTION NOTE University of Illinois at Urbana-Champaign Library Large-scale Digitization Project, 2007. CI u/l Natural History Survey cF Library (/4(I) ILLINOIS NATURAL HISTORY OT TSrX O IJX6V E• The Round Goby (Neogobius melanostomus):A Review of European and North American Literature with notes from the Round Goby Conference, Chicago, 1996 Center for Aquatic Ecology J. Ei!en Marsden, Patrice Charlebois', Kirby Wolfe Illinois Natural History Survey and 'Illinois-Indiana Sea Grant Lake Michigan Biological Station 400 17th St., Zion IL 60099 David Jude University of Michigan, Great Lakes Research Division 3107 Institute of Science & Technology Ann Arbor MI 48109 and Svetlana Rudnicka Institute of Fisheries Varna, Bulgaria Illinois Natural History Survey Lake Michigan Biological Station 400 17th Sti Zion, Illinois 6 Aquatic Ecology Technical Report 96/10 The Round Goby (Neogobius melanostomus): A Review of European and North American Literature with Notes from the Round Goby Conference, Chicago, 1996 J. Ellen Marsden, Patrice Charlebois1, Kirby Wolfe Illinois Natural History Survey and 'Illinois-Indiana Sea Grant Lake Michigan Biological Station 400 17th St., Zion IL 60099 David Jude University of Michigan, Great Lakes Research Division 3107 Institute of Science & Technology Ann Arbor MI 48109 and Svetlana Rudnicka Institute of Fisheries Varna, Bulgaria The Round Goby Conference, held on Feb. 21-22, 1996, was sponsored by the Illinois-Indiana Sea Grant Program, and organized by the -

ARIID Ariu 29 1983 FAO SPECIES IDENTIFICATION SHEETS FAMILY
click for previous page ARIID Ariu 29 1983 FAO SPECIES IDENTIFICATION SHEETS FAMILY: ARIIDAE FISHING AREA 51 (W. Indian Ocean) Arius sumatranus Bennett, 1830 OTHER SCIENTIFIC NAMES STILL IN USE: Tachysurus sumatranus (Bennett, 1830) VERNACULAR NAMES: FAO : En - Goat catfish Fr - Mâchoiron surmulet Sp - Bagre chivato NATIONAL : DISTINCTIVE CHARACTERS: Dorsal profile of head as a gentle slope to first dorsal fin base; 3 pairs of barbels around mouth, the maxillary pair extending to pectoral fin base; head shield thinly granulated and rugose on occipital region only; supraoccipital process keeled, as long as broad at base, its hind end emarginate; median longitudinal groove narrow, reaching to base of supraoccipital process; predorsal plate U-shaped; premaxillary band of teeth in upper jaw 5 times as long as broad, mandibulary band of teeth in lower jaw deeply curved and medially interrupted, palate teeth villïform, in a single ovate patch on each side, sparsely packed. and well separated. First dorsal and pectoral fins each with a strong spine; total anal fin rays 18 to 23. Colour: dark brown above becoming lighter on sides and on belly; fins grey-edged, adipose fin greyish. DISTINGUISHING CHARACTERS OF SIMILAR SPECIES OCCURRING IN THE AREA: snout long Arius caelatus: palate tooth patches much and spatulate larger than in A. sumatranus, triangular and densely maxillary packed. barbel short A. platystomus: palate tooth patches large, snout duck-bill shaped oval, and more densely packed; snout duck-bill shaped. A. subrostratus: palate tooth patches with densely packed teeth; snout long, spatulate, maxil- lary barbels usually not extending beyond eye. A. arius, A. -

Inland Fishes of California
Inland Fishes of California Revise d and Expanded PETER B. MO YL E Illustrations by Chris Ma ri van Dyck and Joe Tome ller i NIVERS ITyor ALfFORNJA PRESS Ikrkd cr I.", ..\ n~d e ' Lon don Universit }, 0 Ca lifornia Press Herkdey and Los Angeles, Ca lifornia Uni ve rsity of alifornia Press, Ltd. Lundun, England ~ 2002 by the Regents of the Unive rsi ty of Ca lifornia Library of Cungress ataloging-in -Publ ica tion Data j\·[oyk, Pen: r B. Inland fis hes of California / Peter B. Moyle ; illustrations by Chris Mari van D)'ck and Joe Tomell eri.- Rev. and expanded. p. cm. In lu de> bibl iographical refe rences (p. ). ISBN 0- 20-2.2754 -'1 (cl ot h: alk. papa) I. rreshw:ltcr lishes-Cali(ornia. I. Title. QL62S C2 M6H 2002 597 .17/i'097Q4-dc21 20010 27680 1\!UlIl.Ifaclu rcd in Canacla II 10 Q9 00 07 06 0 04 03 02 10 ' 1\ 7 b '; -\ 3 2 1 Th paper u!)ed in thi> public.ltiu(] 111l'd., the minimum requirements "fA SI / i': ISO Z39.4H-1992 (R 199;) ( Pmlllllh'/l e ofPa pcr) . e Special Thanks The illustrations for this book were made possible by gra nts from the following : California-Nevada Chapter, American Fi she ries Soc iety Western Di vision, Am erican Fi she ries Society California Department of Fish and Game Giles W. and El ise G. Mead Foundation We appreciate the generous funding support toward the publication of this book by the United Sta tes Environme ntal Protection Agency, Region IX, San Francisco Contents Pre(acc ix Salmon and Trout, Salmonidae 242 Ackl10 11'ledgl11 el1ts Xlll Silversides, Atherinopsidae 307 COlll'er,<iol1 ['actors xv -

DNA Barcoding of Gobiid Fishes (Perciformes, Gobioidei)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository http://informahealthcare.com/mdn ISSN: 1940-1736 (print), 1940-1744 (electronic) Mitochondrial DNA, Early Online: 1–5 ! 2013 Informa UK Ltd. DOI: 10.3109/19401736.2013.834438 SHORT COMMUNICATION DNA barcoding of gobiid fishes (Perciformes, Gobioidei) Divya Viswambharan1, A. Pavan-Kumar1, Dhirendra P. Singh1, A. K. Jaiswar1, S. K. Chakraborty1, J. Rajashekharan Nair2, and W. S. Lakra1 1Division of Fish Genetics and Biotechnology, Central Institute of Fisheries Education, Mumbai, Maharashtra, India and 2Department of Fishery Biology, Kerala University of Fisheries and Ocean Studies, Kochi, Kerala, India Abstract Keywords Gobiids constitute a major proportion of fish population in both tropical and temperate Cytochrome c oxidase subunit I, DNA freshwater as well as marine ecosystem. Due to their small size, cryptic ecology and ambiguous barcoding, gobiid fishes, nucleotide morphological characters, gobiids diversity was not documented completely. In this study, DNA diagnostic characters barcodes were generated for 11 species of gobiids, collected from the Ashtamudi Lake, India. The mitochondrial COI gene was amplified using universal primers and the resulted 650 bp History amplicon was sequenced. The COI barcodes clearly distinguished all the species with high inter- specific genetic distance values than intra-specific values based on K2P (Kimura 2 Parameter) Received 2 May 2013 model. The average genetic distance (K2P model) within species, genus and family was 1.2%, Revised 5 August 2013 22.2% and 25.3%, respectively. In addition to barcode-based species identification system, Accepted 10 August 2013 Nucleotide Diagnostic (ND) characters specific for species were identified. -

Patterns of Evolution in Gobies (Teleostei: Gobiidae): a Multi-Scale Phylogenetic Investigation
PATTERNS OF EVOLUTION IN GOBIES (TELEOSTEI: GOBIIDAE): A MULTI-SCALE PHYLOGENETIC INVESTIGATION A Dissertation by LUKE MICHAEL TORNABENE BS, Hofstra University, 2007 MS, Texas A&M University-Corpus Christi, 2010 Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY in MARINE BIOLOGY Texas A&M University-Corpus Christi Corpus Christi, Texas December 2014 © Luke Michael Tornabene All Rights Reserved December 2014 PATTERNS OF EVOLUTION IN GOBIES (TELEOSTEI: GOBIIDAE): A MULTI-SCALE PHYLOGENETIC INVESTIGATION A Dissertation by LUKE MICHAEL TORNABENE This dissertation meets the standards for scope and quality of Texas A&M University-Corpus Christi and is hereby approved. Frank L. Pezold, PhD Chris Bird, PhD Chair Committee Member Kevin W. Conway, PhD James D. Hogan, PhD Committee Member Committee Member Lea-Der Chen, PhD Graduate Faculty Representative December 2014 ABSTRACT The family of fishes commonly known as gobies (Teleostei: Gobiidae) is one of the most diverse lineages of vertebrates in the world. With more than 1700 species of gobies spread among more than 200 genera, gobies are the most species-rich family of marine fishes. Gobies can be found in nearly every aquatic habitat on earth, and are often the most diverse and numerically abundant fishes in tropical and subtropical habitats, especially coral reefs. Their remarkable taxonomic, morphological and ecological diversity make them an ideal model group for studying the processes driving taxonomic and phenotypic diversification in aquatic vertebrates. Unfortunately the phylogenetic relationships of many groups of gobies are poorly resolved, obscuring our understanding of the evolution of their ecological diversity. This dissertation is a multi-scale phylogenetic study that aims to clarify phylogenetic relationships across the Gobiidae and demonstrate the utility of this family for studies of macroevolution and speciation at multiple evolutionary timescales.