Adsorption of Chlorhexidine Digluconate on Acid Modified Fly Ash: Kinetics, Isotherms and Influencing Factors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tourist Places in and Around Dhanbad
Tourist Places in and around Dhanbad Dhanbad the coal capital of India lies at the western part of Eastern Indian Shield, the Dhanbad district is ornamented by several tourist spots, namely Parasnath Hill, Parasnath Temple, Topchanchi, famous Jharia coalfields, to mention a few. Other important places are Bodh Gaya, Maithon Dam, and this town is only at 260 km distance by rail route from Kolkata. Bodh Gaya Lying at 220 km distance from Dhanbad. Bodh Gaya is the place where Gautam Buddha attained unsurpassed, supreme Enlightenment. It is a place which should be visited or seen by a person of devotion and which would cause awareness and apprehension of the nature of impermanence. About 250 years after the Enlightenment, the Buddhist Emperor, Ashoka visited the site of pilgrimage and established the Mahabodhi temple. Parasnath Temple The Parasnath Temple is considered to be one of the most important and sanctified holy places of the Jains. According to Jain tradition, no less than 23 out of 24 Tirthankaras (including Parsvanatha) are believed to have attained salvation here. Baidyanath Temple Baidyanath Jyotirlinga temple, also known as Baba dham and Baidyanath dham is one of the twelve Jyotirlingas, the most sacred abodes of Shiva. It is located in Deoghar at a distance of 134 km from Dhanbad. It is a temple complex consisting of the main temple of Baba Baidyanath, where the Jyotirlinga is installed, and 21 other temples. Maithon Dam Maithon is 52 km from Dhanbad. This is the biggest reservoir in the Damodar Valley. This dam, designed for flood control, has been built on Barakar river. -

Tata Power: “Renewables to Power Growth” an Exemplar of the Indian Energy Transition
Simon Nicholas, Energy Finance Analyst 1 Tim Buckley, Director of Energy Finance Studies April 2019 Tata Power: “Renewables to Power Growth” An Exemplar of the Indian Energy Transition Executive Summary Tata Power recently made it publicly clear that it will not be building any new coal- fired power capacity going forward. Furthermore, the company has committed to driving the great majority of its power capacity expansion via lower cost renewable energy. The majority of Tata Power’s thermal capacity is centred on its Mundra coal-fired power plant which is financially unviable and making consistent, significant losses that are dragging back the company’s overall financial performance. Since the Mundra plant was commissioned in fiscal year (FY) 2012-13, thermal power has made up only 3% of net capacity additions whilst wind and solar make up 87% and hydro 11%. This represents a significant departure from the accepted wisdom of just a few years ago that a major expansion of coal-fired power would be required to serve India’s growing electricity demand. Figure 1: Renewables and Hydro Will Dominate Tata Power’s Future Capacity Additions (MW) Source: Tata Power Strategic Intent 2025. Tata Power: “Renewables to Power Growth” 2 Tata Power’s shift mirrors the transition underway within the Indian power sector as a whole, driven by least cost renewable energy. Over the first 11 months of FY2018- 19, only 20 megawatts (MW) (net) Tata Power’s shift mirrors of thermal power has been added in the transition underway within India after taking closures into account. Renewable energy the Indian power sector, driven additions over the same period by least cost renewable energy. -

Tata Power (TATPOW) | 71 Target : | 79 Target Period : 12 Months Potential Upside : 12% All Round Performance …
Result Update May 25, 2016 Rating matrix Rating : Buy Tata Power (TATPOW) | 71 Target : | 79 Target Period : 12 months Potential Upside : 12% All round performance … • Revenues came in at | 9374 crore up 13.8% YoY, higher that I-Direct What’s changed? estimates of | 9037 crore. On a segmental basis, power segment Target Changed from | 67 to | 79 revenues were higher that estimates at | 7025 crore (estimate of EPS FY17E Changed from | 5.3 to | 4.6 |6624 crore) while coal segment revenues came in line at | 2023 EPS FY18E Changed from | 5.8 to | 5.1 crore vs. estimate of |2000 crore. Rating Changed from Hold to Buy • Performance across subsidiaries was reasonably strong. The likes of Maithon power (strong generation), Mundra (lower fuel costs), Tata Quarterly performance Solar (strong revenue booking and outlook) added to overall Q4FY16 Q4FY15 YoY (%) Q3FY16 QoQ (%) performance. Revenue 9333 8240 13 8952 4 • EBITDA margins came in at 20.4% for Q4FY16 vs. our estimate of EBITDA 1913 1962 (3) 2095 (9) 23% for the quarter. The key miss stemmed due to miss in the power EBITDA (%) 20 24 (331) 23 (291) segment EBIT which came in at 17.3% YoY as compared to 19.4% in PAT 356 (13) 2884 260 37 Q4FY15. The same is reiterated from the fuel costs which have gone up sharply by 73% YoY Key financials • Higher than expected revenues, strong subsidiary performance and | Crore FY15 FY16 FY17E FY18E one off regulatory gain led to a PAT of |360 crore higher than our Net Sales 34366.9 37480.2 39642.3 41154.6 estimates of |320 crore in Q4FY16 . -

Access Jharkhand-Obj07-04-2021-E-Book
Index 01. Jharkhand Special Branch Constable (Close 16. JSSC Assistant Competitive Examination Cadre) Competitive Exam 01-09-2019 28.06.2015. 02. J.S.S.C. - Jharkhand Excise Constable Exam 17. Jharkhand Forest Guard Appointment Com- 04-08-2019 petitive (Prelims) Exam - 24.05.2015. 03. SSC IS (CKHT)-2017, Intermediate Level (For 18. Jharkhand Staff Selection Commission the post of Hindi Typing Noncommittee in Com- organized Women Supervisor competitive puter Knowledge and Computer) Joint Competi- Exam - 2014. tive Exam 19. Fifth Combined Civil Service Prelims Compet- 04. JUVNL Office Assistent Exam 10-03-2017 itive Exam - 15.12.2013. 05. J.S.S.C. - Post Graduate Exam 19-02-2017 20. Jharkhand Joint Secretariat Assistant (Mains) 06. J.S.S.C Amin Civil Resional Investigator Exam Examination 16.12.2012. 08-01-2017 21. State High School Teacher Appointment 07. JPSC Prelims Paper II (18.12.2016) Examination 29.08.2012. 08. JPSC Prelims Paper-I (Jharkhand Related 22. Jharkhand Limited Departmental Exam- Questions Only on 18.12.2016) 2012. 09. Combined Graduation Standard Competitive 23. Jharkhand Joint Secretariat Assistant Exam- (Prelims) Examinations 21.08.2016 2012. 10. Kakshpal appointment (mains) Competitive 24. Fourth Combined Civil Service (Prelims) Examination 10.07.2016. Competitive Examination - 2010. 11. Jharkhand Forest guard appointment (mains) 25. Government High School Teacher Appoint- Competitive Examination 16.05.2016. ment Exam - 2009. 12. JSSC Kakshpal Competitive (Prelims) Exam - 26. Primary Teacher Appointment Exam - 2008. 20.03.2016. 27. Third Combined Civil Service Prelims 13. Jharkhand Police Competitive Examination Competitive Exam - 2008. 30.01.2016. 28. JPSC Subsidiary Examination - 2007. -

National Mega & Ultra Mega Power Plants
NATIONAL MEGA & ULTRA MEGA POWER PLANTS • Mega Power Plant- According to a circular issued by the Government of India dated 10th November, 1995 regarding the ‘Setting up of power plants of capacity 1000 MW or more supplying power to more than one State’, Government of India had set the guidelines of setting up the mega power projects of capacity 1000 MW or more supplying power to more than one State had been issued vide D.O. letter N. C.- 286/95-IPC, dated 10th November, 1995. S. No. POWER PLANT STATE COMMISSIONED CAPACITY (MW) COAL BASED THERMAL POWER PLANTS 1. Sipat Thermal Power Station, NTPC Chhattisgarh 2,980 2. Korba Super Thermal Power Plant, Chhattisgarh 2,600 NTPC 3. Rihand Thermal Power Station, NTPC Uttar Pradesh 3,000 4. Singrauli Super Thermal Power Uttar Pradesh 2,000 Trans India Law Associates, Advocates & Legal Consultants Office: TILA SUITE, JA-120, DLF Tower - A, Jasola District Center, New Delhi-110025 Chamber: TILA Chamber No. 555, New Delhi Court Complex, New Delhi-110 001 Tel.: 91-11-26943664, Fax.: 91-11-26970075 Whatsapp: +91 9810070075 [email protected] www.tila.in NEW DELHI | Associates/Consultants all over India & Abroad Bangladesh/ Cameroon/ China/ Myanmar/ Nepal/ Singapore/ South Korea/ UAE Station, NTPC 5. NTPC Dadri Uttar Pradesh 1,820 6. Feroj Gandhi Unchahar Thermal Uttar Pradesh 1,050 Power Plant, NTPC 7. Barh Super Thermal Power Station, Bihar 3,300 NTPC 8. Talcher Super Thermal Power Station, Odisha 3,000 NTPC 9. Mejia Thermal Power Station, DVC West Bengal 2,340 10. Kahalgaon Super Thermal Power Bihar 2,340 Station, NTPC 11. -

14February 2017 India Daily
INDIA DAILY February 14, 2017 India 13-Feb 1-day 1-mo 3-mo Sensex 28,352 0.1 4.1 5.7 Nifty 8,805 0.1 4.8 6.1 Contents Global/Regional indices Dow Jones 20,412 0.7 2.6 8.2 Special Reports Nasdaq Composite 5,764 0.5 3.4 10.5 Strategy FTSE 7,279 0.3 (0.8) 7.8 Nikkei 19,452 (0.0) 0.9 10.0 Strategy: Day 1 takeaways from Chasing Growth 2017 Hang Seng 23,711 0.6 3.4 6.7 Daily Alerts KOSPI 2,076 (0.1) (0.0) 5.1 Value traded – India Results Cash (NSE+BSE) 232 260 119 Motherson Sumi Systems: SMRPBV growth still below par but standalone Derivatives (NSE) 3,613 2,813 4,776 showing strong growth Deri. open interest 3,170 2,806 2,814 NMDC: Weak due to one-offs Forex/money market IDEA: Weak earnings print on expected lines Change, basis points Hindalco Industries: In-line quarter; demonetization impacts marginally 13-Feb 1-day 1-mo 3-mo Rs/US$ 66.9 (5) (118) (95) Tata Power: Underlying strength, distorted by one-offs 10yr govt bond, % 7.3 3 49 26 Muthoot Finance: Strong performance in a challenging environment Net investment (US$ mn) 10-Feb MTD CYTD National Aluminium Co.: Alumina prices aid improvement (1,170 FIIs 83 2,903 ) Balkrishna Industries: Robust quarter MFs (2) 881 6,951 Top movers SRF: Waiting for turnaround Change, % Prestige Estates Projects: Demonetization leads to sales collapse; but Best performers 13-Feb 1-day 1-mo 3-mo collections from past sales sustain IDEA IN Equity 107.3 (2.9) 55.9 53.3 Jagran Prakashan: Steady quarter JPA IN Equity 13.2 (1.9) 33.5 50.3 HPCL IN Equity 578.3 1.2 18.3 28.9 Company alerts TTAN IN Equity 428.4 (0.8) 19.5 27.9 DLFU IN Equity 147.8 (0.1) 19.1 26.9 Infosys: Takeaways from CEO's address at Kotak conference: Expect stable growth Worst performers DIVI IN Equity 740.4 (1.3) (0.8) (39.4) Economy alerts RCOM IN Equity 33.6 (3.0) 7.0 (19.7) Economy: Core inflation inches higher CRG IN Equity 66.8 (6.2) 3.8 (15.1) ARBP IN Equity 662.0 (2.6) (6.0) (14.3) UBBL IN Equity 785.1 (0.8) (5.1) (10.0) For Private Circulation Only. -

India's Leading Infrastructure Companies 2018
NaxtGan Infra: Building India 0'Tomorrow GROWING RELATIONSHIPS THROUGH DATA India's Leading& Bradstreet InfrastructureDun Companies 2018 I & Bradstreet Dun India’s Leading Infrastructure & Bradstreet DunCompanies 2018 & Bradstreet Dun India’s Leading Infrastructure & Bradstreet DunCompanies 2018 India’s Leading Infrastructure Companies 2018 Published in India by Dun & Bradstreet Information Services India Pvt Ltd. Registered Office New Delhi Office ICC Chambers, Saki Vihar Road, 1st Floor, Administrative Building, Powai, Mumbai - 400072. Block ‘E’, NSIC - Technical Services Center, CIN: U74140MH1997PTC107813 Okhla Industrial Estate Phase - III, Tel: +91 22 6676 5555 / 2857 4190 / 92 / 94 New Delhi - 110020. +91 22 6680 1300 / 2847 6291 / 93 Tel: +91 11 4149 7900 / 01 Email: [email protected] Fax: +91 11 4149 7902 URL: www.dnb.co.in Kolkata Office Chennai Office Ahmedabad Office 166B, S. P. Mukherjee Road, New No: 28, Old No: 195, 801 - 8th Floor, Shapath V, Merlin Links, Unit 3E, 3rd Floor, 1st Floor, North Usman Road, Opp. Karnavati Club, Kolkata - 700026. T. Nagar, Chennai - 600017. S. G. Highway, Tel: +91 33 2465 0204 Tel: +91 44 4289 7602 / 7619 Ahmedabad - 380054. Fax: +91 33 2465 0205 Fax: +91 44 2814 2285 Tel: +91 79 4900 4601 / 602 Bengaluru Office Hyderabad Office Haryana Office No. 7/2 Gajanana Towers, The Executive Centre, Level 1, Unit 2, 706, 7th Floor, Tower B, 1st Floor, Annaswamy Mudaliar Street, Salarpuria Sattva Knowledge City, Global Business Park, Opp. Ulsoor Lake, Inorbit Mall Road, Mehrauli Gurgaon -

Owner: Maithon Power Limited Project Manager: the Tata
OWNER: MAITHON POWER LIMITED PROJECT MANAGER: THE TATA POWER COMPANY LIMITED PROJECT: COAL SAMPLING AND ANALYSIS AT 2 X 525 MW MAITHON POWER LIMITED (MPL), JHARKHAND - INDIA ENQUIRY REFERENCE NO.: CC20KNC005 - COAL SAMPLING AND ANALYSIS EXPRESSION OF INTEREST (EOI) COAL SAMPLING AND ANALYSIS FOR MAITHON POWER LIMITED Enquiry reference no.: CC20KNC005 - COAL SAMPLING AND ANALYSIS Name of Package: COAL SAMPLING AND ANALYSIS at 2 X 525 MW Maithon Power Limited (MPL), Jharkhand – India Type of Bidding: E-tendering / Two Part Contact Details: Mr Nagdhar C Kandi The Tata Power Company Limited, Smart Center of Procurement Excellence, 2nd Floor, Sahar Receiving Station, Near Hotel Leela, Sahar Airport Road, Andheri East, Mumbai-400059, Maharashtra, India Email: Email: [email protected] Tel: +91 22 6717 3941 (L), + 91 9223320928 (M) Website: https://www.tatapower.com/tender/tenderlist.aspx The Tata Power Company Limited (“Project Manager”) on behalf of M/s Maithon Power Limited (“Owner”) invites Expression of Interest (EOI) from interested parties for the Two-Part e-Tendering Process of following Relevant Package: Description Bid Security Tender Fee (EMD) Services required for the period of three years from independent inspection agency (Service Providers) to assess, verify and cross check the quality of coal at loading points i.e. at mine’s end and at unloading point i.e. at MPL Coal Yard.( MPL shall appoint more INR 10 lakhs INR 1,000/- than one agency and the agency at the loading end and unloading ends would be different) for 2x525 MW units at Maithon Power Limited 1. INTRODUCTION: The Tata Power Company Limited (TPC) is among the largest private sector Power Utility companies in India with presence in Generation, Transmission and Distribution of Power through conventional and renewable sources. -
S.No. District Code Name of the Establishment Address Major
Jharkhand S.No. District Name of the Address Major Activity Broad NIC Owners Employ Code Establishment Description Activity hip ment Code Code Class Interval 1 01 Madhya vidhalya sisari 822114 Education 20 851 1 15-19 BOKARO STEEL 2 BHAVNATHPUR Mining 05 051 4 25-29 MINES TOWNSHEEP BHAVNATHPUR TOWNSHEEP 822112 201 VATIKA HOTEL 9 GURUDAWARA Resturant 14 561 2 15-19 GALI GURUDAWARA GALI 815301 304 SAWAN BEAR BAR 19 GANDHI CHOWK Resturant 14 563 2 10-14 GANDHI CHOWK 404 815301 MAHATO HOTEL 103 AURA AURA Hotel 14 562 2 10-14 504 825322 6 04 HOTEL KALPANA 19 ISRI ISRI 825107 Resturant 14 561 2 15-19 7 04 HOTEL KAVERI 64 ISRI ISRI 825107 Resturant 14 561 2 10-14 HARIDEVI REFRAL 89 THAKURGANGTI Health 21 861 1 10-14 806HOSPITAL 813208 RAJMAHAL 105 814154 Health 21 861 4 30-99 PARIYOJNA 906HOSPITAL SAMUDAYIK HEALTH PATHERGAMA 814147 Health 21 861 1 30-99 10 06 CENTER SAMUDYIK HEALTH 129 SUNDERPAHARI Health 21 861 1 15-19 CENTER 814133 11 06 rajkiya madh vidyalaya 835302 Education 20 851 1 15-19 12 11 jeema ICICI BANK 160 RAMGARH Banking 16 641 2 10-14 13 16 829118 PRATHMIK BLOCK MOD Health 21 861 1 15-19 SWASTHYA KENDRA PATRATU 829118 14 16 CCL HOSPITAL 82 RAMGARH 829106 Health 21 871 1 30-99 15 16 BHURKUNDA JINDAL STEEL AND 4(1) PATRATU Manufature 06 243 4 >=500 16 16 POWER BALKUDRA 829118 KEDLA WASHRI BASANT PUR Mining 05 051 2 >=500 17 16 WASHRI 829101 PRERNA MAHILA 126(2) SANGH Retail 12 472 5 10-14 VIKASH MANDAL RAMNAGAR BARKA CHUMBA 18 16 RAMNAGAR 829101 BIRU TASHA PARTY 89(2) BARKA Exitment 19 772 2 15-19 CHUMBA BRAHMAN 19 16 MUHALLA 829101 -

Industrial Interaction
Consultancy Projects 2010-11 Consultancy Projects commenced during Financial Year 2010-11: Sl. Consultancy Title of the Consultancy Project Consultant In- Sponsoring No. Project Charge Organization Number 1. 1027 Analysis of ETP sludge from Khadia project of Prof. Gurdeep NCL NCL Singh 2. 1051 Testing of Drinking water sample from Bhuli Prof. Gurdeep BCCL Treatment Plant, BCCL Singh 3. 1080 Monitoring of environmental parameters in Prof. Gurdeep BTPS respect of ambient air, stack monitoring, noise Singh level and effluent, BTPS’B’ Bokaro Thermal, Bokaro 4. 1082 Baseline Data Generation for preparation of Prof. Gurdeep BCCL EIA/EMP of Damuda Group of cluster-I and Singh Madhuband NLW Washery 5 MTY, BCCL, Dhanbad 5. 1083 Baseline Data generation for preparation Prof. Gurdeep BCCL EIA/EMP of Madhuband NLW Coal Washery (5 Singh MT PA) as per TOR granted by MoEF. of Koyla Nagar, Dhanbad 6. 1091 Design of Particulates Emission Control System Dr. M. K. Jain BBCL and preparation of environmental management plan for coke oven plants in Dhanbad 7. 1093 Preparation of Environmental Statement Report of Prof. Gurdeep DVC DVC, Bermo Mines, Bermo, Bokaro Singh 8. 1094 Effect of Ash from captive power plant Kathara Prof. Gurdeep CCL Area, CCL Singh 9. 1095 Analysis of ground water sample for As, Fe & Mn Prof. Gurdeep CGWB of 86 nos. sample of Central Ground Water Board Singh Patna 10. 1096 Design of Particulate emission control system and Dr. S. K. Gupta SSCMIPL preparation of environmental management plant for coke oven plants in Dhanbad 11. 1097 Testing of Dust suppressor chemical under RCRA Prof. -

2020 India MAPPING-1 NCERT BASED
MAPPING MADE EASY -2020 India MAPPING-1 NCERT BASED Table of Contents S.no. Title Page India– Location 2 1 Structure and physiography 4 2 Drainage system 17 3 Climate 24 4 Natural vegetation 52 5 Soils 56 6 1 Page Aspire IASThe name associated with excellence 10/70 Old Rajeneder Nagar N.Delhi www.aspireias.com 8010068998/9999801394 ©2020 ASPIRE IAS. All rights reserved MAPPING MADE EASY -2020 India MAPPING-1 NCERT BASED due to their vast east-to-west extent. For 1. India– Location example, the USA has seven time zones. India, extends from Kashmir in the north to Size Kanniyakumari in the south and Arunachal Pradesh in the east to Gujarat in the west. India‘s Indian subcontinent- territorial limit further extends towards the sea • upto 12 nautical miles (about 21.9 km) from the It includes the countries — Pakistan, coast. Nepal, Bhutan, Bangladesh and India. • The Himalayas, together with other ranges, • Southern boundary extends upto 6°45' N have acted as a formidable physical barrier latitude in the Bay of Bengal. in the past. Except for a few mountain • the latitudinal and longitudinal extent of passes such as the Khyber, the Bolan, the India, they are roughly about 30 degrees, Shipkila, the Nathula, the Bomdila, etc. it whereas the actual distance measured from was difficult to cross it. north to south extremity is 3,214 km, and • Peninsular part of India extends towards that from east to west is only 2,933 km. the Indian Ocean. (provided the country • distance between two longitudes decreases with a coastline of 6,100 km in the towards the poles whereas the distance mainland and 7,517 km in the entire between two latitudes remains the same geographical coast of the mainland plus everywhere. -
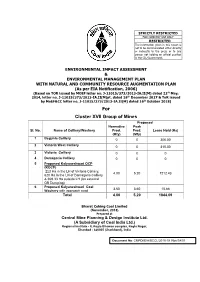
For Cluster XVII Group of Mines from Ministry of Environment & Forests, Govt
STRICTLY RESTRICTED FOR COMPANY USE ONLY RESTRICTED The information given in this report is not to be communicated either directly or indirectly to the press or to any person not holding an official position in the CIL/Government. ENVIRONMENTAL IMPACT ASSESSMENT & ENVIRONMENTAL MANAGEMENT PLAN WITH NATURAL AND COMMUNITY RESOURCE AUGMENTATION PLAN (As per EIA Notification, 2006) (Based on TOR issued by MOEF letter no. J-11015/373/2013-IA.II(M) dated 21St May, 2014, letter no. J-11015/373/2013-IA.II(M)pt. dated 26th December 2017 & ToR issued by MoEF&CC letter no. J-11015/373/2013-IA.II(M) dated 16th October 2018) For Cluster XVII Group of Mines Proposed Normative Peak Sl. No. Name of Colliery/Washery Prod. Prod. Lease Hold (Ha) (Mty) (Mty) 1 Begunia Colliery 0 0 306.00 2 Victoria West Colliery 0 0 310.00 3 Victoria Colliery 0 0 0 4 Damagoria Colliery 0 0 0 5 Proposed Kalyaneshwari OCP (KOCP) 223 Ha in the LH of Victoria Colliery, 4.00 5.20 1212.43 620 Ha in the LH of Damagoria Colliery & 369.33 Ha outside LH (for external OB Dumping) 6 Proposed Kalyaneshwari Coal 3.60 3.60 15.66 Washery with approach road Total 4.00 5.20 1844.09 Bharat Coking Coal Limited (November, 2018) Prepared at Central Mine Planning & Design Institute Ltd. (A Subsidiary of Coal India Ltd.) Regional Institute - II, Koyla Bhawan complex, Koyla Nagar, Dhanbad - 826005 (Jharkhand), India Document No: CMPDI/EIA/BCCL/2018-19 /Nov/54/01 C O N T E N T S CHAPTER NO.