Effects of Aqueous Extract of Pausinystalia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alkaloids Used As Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look
molecules Review Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look Michael Heinrich 1,2,* , Jeffrey Mah 1 and Vafa Amirkia 1 1 Research Group ‘Pharmacognosy and Phytotherapy’, UCL School of Pharmacy, University of London, 29–39 Brunswick Sq., London WC1N 1AX, UK; [email protected] (J.M.); [email protected] (V.A.) 2 Graduate Institute of Integrated Medicine, College of Chinese Medicine, and Chinese Medicine Research Center, China Medical University, No. 100, Section 1, Jingmao Road, Beitun District, Taichung 406040, Taiwan * Correspondence: [email protected]; Tel.: +44-20-7753-5844 Abstract: Selecting candidates for drug developments using computational design and empirical rules has resulted in a broad discussion about their success. In a previous study, we had shown that a species’ abundance [as expressed by the GBIF (Global Biodiversity Information Facility)] dataset is a core determinant for the development of a natural product into a medicine. Our overarching aim is to understand the unique requirements for natural product-based drug development. Web of Science was queried for research on alkaloids in combination with plant systematics/taxonomy. All alkaloids containing species demonstrated an average increase of 8.66 in GBIF occurrences between 2014 and 2020. Medicinal Species with alkaloids show higher abundance compared to non-medicinal alkaloids, often linked also to cultivation. Alkaloids with high biodiversity are often simple alkaloids found in multiple species with the presence of ’driver species‘ and are more likely to be included in early-stage drug development compared to ‘rare’ alkaloids. Similarly, the success of an alkaloid Citation: Heinrich, M.; Mah, J.; Amirkia, V. -

South Cameroon)
Plant Ecology and Evolution 152 (1): 8–29, 2019 https://doi.org/10.5091/plecevo.2019.1547 CHECKLIST Mine versus Wild: a plant conservation checklist of the rich Iron-Ore Ngovayang Massif Area (South Cameroon) Vincent Droissart1,2,3,8,*, Olivier Lachenaud3,4, Gilles Dauby1,5, Steven Dessein4, Gyslène Kamdem6, Charlemagne Nguembou K.6, Murielle Simo-Droissart6, Tariq Stévart2,3,4, Hermann Taedoumg6,7 & Bonaventure Sonké2,3,6,8 1AMAP Lab, IRD, CIRAD, CNRS, INRA, Université de Montpellier, Montpellier, France 2Missouri Botanical Garden, Africa and Madagascar Department, P.O. Box 299, St. Louis, Missouri 63166-0299, U.S.A. 3Herbarium et Bibliothèque de Botanique africaine, C.P. 265, Université Libre de Bruxelles, Campus de la Plaine, Boulevard du Triomphe, BE-1050 Brussels, Belgium 4Meise Botanic Garden, Domein van Bouchout, Nieuwelaan 38, BE-1860 Meise, Belgium 5Evolutionary Biology and Ecology, Faculté des Sciences, C.P. 160/12, Université Libre de Bruxelles, 50 Avenue F. Roosevelt, BE-1050 Brussels, Belgium 6Plant Systematics and Ecology Laboratory, Higher Teachers’ Training College, University of Yaoundé I, P.O. Box 047, Yaoundé, Cameroon 7Bioversity International, P.O. Box 2008 Messa, Yaoundé, Cameroon 8International Joint Laboratory DYCOFAC, IRD-UYI-IRGM, BP1857, Yaoundé, Cameroon *Author for correspondence: [email protected] Background and aims – The rapid expansion of human activities in South Cameroon, particularly mining in mountainous areas, threatens this region’s exceptional biodiversity. To comprehend the effects of land- use change on plant diversity and identify conservation priorities, we aim at providing a first comprehensive plant checklist of the Ngovayang Massif, focusing on the two richest plant families, Orchidaceae and Rubiaceae. -

Natural Products (Secondary Metabolites)
Biochemistry & Molecular Biology of Plants, B. Buchanan, W. Gruissem, R. Jones, Eds. © 2000, American Society of Plant Physiologists CHAPTER 24 Natural Products (Secondary Metabolites) Rodney Croteau Toni M. Kutchan Norman G. Lewis CHAPTER OUTLINE Introduction Introduction Natural products have primary ecological functions. 24.1 Terpenoids 24.2 Synthesis of IPP Plants produce a vast and diverse assortment of organic compounds, 24.3 Prenyltransferase and terpene the great majority of which do not appear to participate directly in synthase reactions growth and development. These substances, traditionally referred to 24.4 Modification of terpenoid as secondary metabolites, often are differentially distributed among skeletons limited taxonomic groups within the plant kingdom. Their functions, 24.5 Toward transgenic terpenoid many of which remain unknown, are being elucidated with increas- production ing frequency. The primary metabolites, in contrast, such as phyto- 24.6 Alkaloids sterols, acyl lipids, nucleotides, amino acids, and organic acids, are 24.7 Alkaloid biosynthesis found in all plants and perform metabolic roles that are essential 24.8 Biotechnological application and usually evident. of alkaloid biosynthesis Although noted for the complexity of their chemical structures research and biosynthetic pathways, natural products have been widely per- 24.9 Phenylpropanoid and ceived as biologically insignificant and have historically received lit- phenylpropanoid-acetate tle attention from most plant biologists. Organic chemists, however, pathway metabolites have long been interested in these novel phytochemicals and have 24.10 Phenylpropanoid and investigated their chemical properties extensively since the 1850s. phenylpropanoid-acetate Studies of natural products stimulated development of the separa- biosynthesis tion techniques, spectroscopic approaches to structure elucidation, and synthetic methodologies that now constitute the foundation of 24.11 Biosynthesis of lignans, lignins, contemporary organic chemistry. -

Tryptophan Derivatives INDOL ALKALOIDS -Secale Cornutum
Tryptophan Derivatives INDOL ALKALOIDS -Secale Cornutum -Faba calabarica -Semen Strychni -Gelsemium nitidum -Radix Rauwolfiae -Cortex Yohimbae -Aspidiospermae -Herba Catharanthi -Herba Vincae -Cortex Chinconae -Harman Alkaloids(-carboline) -Semen Pegani Radix Rauwolfia Rauwolfia serpentina (Apocynaceae) Radix must contain at least 0.15% reserpine- resinnamine group alkaloids. Rauwolf (German Botanist)16th century Rauwolfia serpentina; Used against snake bites and psyclological problems in India. Powdered roots, extract and purified alkaloids are used to control hypertension. Approximately 50 alkaloids are isolated from 25 Rauwolfia species. These alkaloids shows their activity by stimulating the release of serotonin and catecholamines so that sedative and tranquilizer activity occur. Reserpine; Reserpine absolutely lowers the blood pressure. Decreases pulse number with some euphory. Should be used in schizophrenia. Reserpine is a drug that is used for the treatment of high blood pressure, usually in combination with a thiazide diuretic or vasodilator. Large clinical trials have shown that combined treatment with reserpine plus a thiazide diuretic reduces mortality of people with hypertension. Resinnamine; Genetic hypertension (middle level) Deserpidine; 11-demethoxy reserpine (used like reserpine) Deserpidine and reserpine are used with diuretics against hypertension. Reserpine; white/pale yellow, odorless, crystal powder. Sensitive to light (darkens slowly). Commercial reserpine sources: R. serpentina (reserpine + resinnamine) R. micrantha (reserpine + resinnamine) R. tetraphylla (reserpine + deserpidine) R. vomitoria (reserpine) Mostly used species. Reserpine could be used synthetically but not efficient. Preference is natural sources. The Rauwolfia alkaloids reserpine and deserpidine, two alkaloids from Rauwolfia species, have been widely used for their antihypertensive action. Deserpidine is a compound with limited availability from natural sources, and its synthesis from 1 in six steps (41% overall yield) is reported in literature. -

Double-Blind, Placebo-Controlled Safety and Ef®Cacy Trial with Yohimbine Hydrochloride in the Treatment of Nonorganic Erectile Dysfunction
International Journal of Impotence Research (1997) 9, 155±161 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 Double-blind, placebo-controlled safety and ef®cacy trial with yohimbine hydrochloride in the treatment of nonorganic erectile dysfunction H-J Vogt1, P Brandl1, G. Kockott2, JR Schmitz2, MH Wiegand2, J Schadrack3 and M Gierend3 1 Dermatologische Klinik und Poliklinik; 2 Psychiatrische Klinik und Poliklinik, Technische UniversitaÈtMuÈnchen, Munich, Germany; and 3 Medicomp Gesellschaft fuÈr Versuchsplanung und Datenanalyse mbH, Ulm, Germany This double-blind, placebo-controlled clinical trial of yohimbine hydrochloride included 86 patients with erectile dysfunction and without clearly detectable organic or psychologic causes. The patient group ful®lled all entry criteria; 85 of these could be considered for the Safety- respectively 83 for the Intention-to-treat (ITT)-analysis. Yohimbine was administered orally in a dosage of 30mg a day (two 5mg tablets three times daily) for eight weeks. Patients were seen for follow-up after four weeks' treatment, and for a ®nal visit after eight weeks. Ef®cacy evaluation was based on both subjective and objective criteria. Subjective criteria included improvement in sexual desire, sexual satisfaction, frequency of sexual contacts, and quality of erection (penile rigidity) during sexual contact/intercourse. Objective criteria of outcome were based on improvement in penile rigidity determined by use of polysomnography in the sleep laboratory. Overall Yohimbine was found signi®cantly more effective than placebo in terms of response rate: 71 vs 45%. Yohimbine was well-tolerated: Only 7% of patients rated tolerability fair or poor, and most adverse experiences were mild. -

Droissart Et Al Plant Ecol Evo
Mine versus Wild : a plant conservation checklist of the rich Iron-Ore Ngovayang Massif Area (South Cameroon) Vincent Droissart, Olivier Lachenaud, Gilles Dauby, Steven Dessein, Gyslène Kamdem, Charlemagne Nguembou K., Murielle Simo-Droissart, Tariq Stévart, Hermann Taedoumg, Bonaventure Sonké To cite this version: Vincent Droissart, Olivier Lachenaud, Gilles Dauby, Steven Dessein, Gyslène Kamdem, et al.. Mine versus Wild : a plant conservation checklist of the rich Iron-Ore Ngovayang Massif Area (South Cameroon). Plant Ecology and Evolution, Botanic Garden Meise and Royal Botanical Society of Belgium, 2019, 152 (1), pp.8-29. 10.5091/plecevo.2019.1547. hal-02079407 HAL Id: hal-02079407 https://hal.umontpellier.fr/hal-02079407 Submitted on 26 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Plant Ecology and Evolution 152 (1): 8–29, 2019 https://doi.org/10.5091/plecevo.2019.1547 CHECKLIST Mine versus Wild: a plant conservation checklist of the rich Iron-Ore Ngovayang Massif Area (South Cameroon) Vincent Droissart1,2,3,8,*, Olivier Lachenaud3,4, Gilles Dauby1,5, Steven Dessein4, Gyslène Kamdem6, Charlemagne Nguembou K.6, Murielle Simo-Droissart6, Tariq Stévart2,3,4, Hermann Taedoumg6,7 & Bonaventure Sonké2,3,6,8 1AMAP Lab, IRD, CIRAD, CNRS, INRA, Université de Montpellier, Montpellier, France 2Missouri Botanical Garden, Africa and Madagascar Department, P.O. -

Pharmaka, Philtres, and Pheromones Getting High and Getting Off
26 m a p s • v o l u m e X I I n u m b e r 1 • s e x , s p i r i t , a n d p s y c h e d e l i c s 2 0 0 2 Pharmaka, Philtres, and Pheromones Getting High and Getting Off by Jonathan Ott, Director of Applied Psychonautics, Natural Products Co., Xalapa, Veracruz, México that with regard mones (as exemplified by the truffle, amber[gris], plus ants We’ve been told to seduction, and musks); 2) stimulants (betel nuts); and 3) visionary “candy is dandy, but liquor is quicker,” but in truth, rather, inebriants (Cannabis). I will examine hereunder each of these properly selected: “candy makes randy; liquor makes desire three categories of 16th-century Italian philtre-formulations flicker;” or, as Shakespeare’s porter said to Macduff: “[drink] in some detail. provokes the desire but it takes away the performance.” The wines and beers of antiquity, however, which were potent PHEROMONES, infusions of innumerable psychoactive plants often requiring THE QUINTESSENCE OF PHILTRES dilution with water and in which alcohol served rather as Although the truffle might seem out of place here, in fact preservative than inebriating active principle, had already in it was shown in 1981 to be a potent source of androstenol, Shakespeare’s day given way to straight alcoholic beverages, known since 1944 to be a component of boar-testes, and if anything augmented by the soporific and anerotic hops, patented in the early 1970s for use in artificial insemination Humulus lupulus. -

Herbal Therapy for Men with Erectile Dysfunction
REVIEW Herbal therapy for men with erectile dysfunction Bichitra N Nayak PhD1, Harpal S Buttar DVM PhD FICN2 BN Nayak, HS Buttar. Herbal therapy for men with erectile ED is linked to an increased risk for cardiovascular diseases and stroke. dysfunction. Curr Res Cardiol 2015;2(1):30-34. Several orally active drugs (sildenafil, vardenafil, tadalafil, avanafil) are currently prescribed for treating ED to improve the arterial blood flow to Erectile dysfunction (ED) or male impotence is defined as inability of a the penile tissue. Medicinal plants and their extracts have been used in man to achieve or maintain penile erection sufficient for sexual activity. traditional medicine in southwest Asia and other countries to treat ED. It is primarily a neuronal and endothelial dysfunction of the corpus cav- The current review focuses on four medicinal plants that have been used ernosum of penile tissue, and is partly characterized by reduced produc- as aphrodisiacs for enhancing sexual performance and for the treatment tion of nitric oxide (NO). Other factors that may contribute to the of ED. These plants include Eurycoma longifolia Jack (tongkat ali); pathogenesis of ED include androgen deficiency in aging men, hyperten- Chlorophytum borivilianum (safed musli); Withania somnifera (ashwa- sion, high cholesterol levels, atherosclerosis, diabetes mellitus, diseases of gandha); and Pausinystalia johimbe (formerly known as Corynanthe the prostate and heart, and anatomical deformity of the penis. ED may johimbe). Suggested mechanisms of action for each of the plant extracts also be caused by some medications, prostate surgery and spinal cord will be discussed. injury. Psychological and social conditions such as stress, depression and unhappy marital relationship may contribute to the problem. -
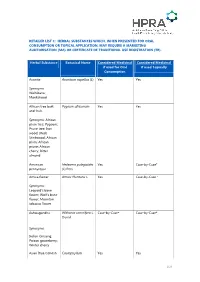
Retailer List C
RETAILER LIST C: HERBAL SUBSTANCES WHICH, WHEN PRESENTED FOR ORAL CONSUMPTION OR TOPICAL APPLICATION, MAY REQUIRE A MARKETING AUHTORISATION (MA) OR CERTIFICATE OF TRADITIONAL USE REGISTRATION (TR). Herbal Substance Botanical Name Considered Medicinal Considered Medicinal if used for Oral if used Topically Consumption Aconite Aconitum napellus (L) Yes Yes Synonyms: Wolfsbane; Monkshood African tree bark Pygeum africanum Yes Yes and fruit. Synonyms: African plum tree; Pygeum; Prune tree; Iron wood; (Red) Stinkwood; African plum; African prune; African cherry; Bitter almond American Hedeoma pulegioides Yes Case-by-Case* pennyroyal (L) Pers Arnica flower Arnica Montana L Yes Case-by-Case * Synonyms: Leopard's bane flower; Wolf's bane flower; Mountain tobacco flower Ashwagandha Withania somnifera L. Case-by-Case* Case-by-Case* Dunal Synonyms: Indian Ginseng; Poison gooseberry; Winter cherry Asian Blue Cohosh Caulophyllum Yes Yes 1/23 Herbal Substance Botanical Name Considered Medicinal Considered Medicinal if used for Oral if used Topically Consumption robustum Maxim Autumn crocus Colchicum autumnale Yes Yes L Synonyms: Meadow Saffron Autumn mandrake Mandragora Yes Yes autumnalis Barbados Aloes Aloe barbadensis If anthraquinone/aloin No: unless medicinal Miller content < 1 ppm then claims are made. Synonyms: (Synonym A.vera L.) not a medicinal Aloe vera product unless medicinal claims are made Barberry Berberis vulgaris Yes Case-by-Case* Synonyms: European barberry; Berberis Bearberry Leaf Arctostaphylos uva- Yes No: unless medicinal ursi claims are made. L. Spreng Synonyms: Uva-Ursi Belladonna herb Atropa belladonna L Yes Yes and root (See list B entry 3) Synonyms: Deadly nightshade Birch leaf and bark Betula pendula ROTH Yes No: unless medicinal claims are made or the essential oil is used. -

Medicinal Plants
MEDICINAL PLANTS Species Name FAMILY NAME Local Names Common Names Parts Used Medicinal Use(S) Fevers, gonorrhoea, dysentery, catarrhal 1 Abelmoschus esculentus Malvaceae Ila,okweje,kubewa Okra,lady's finger Fruit, seeds infections,emollient,antispasmodic, tonic. Oju- ologbo,omisinminsin,mese Colds,cough, convulsion, rheumatism, Crab’s eye,rosary 2 Abrus precatorius Leguminosae nmesen,iwere-jeje,Olorun Root, leaves,seeds conjunctivitis,contraceptive,antimicrobials,aphrodisiac,ulcer,anae pea,love nut,jequirity yin- mia,antidote poison ni,otoberebere,idonzakara 3 Abutilon mauritianum Malvaceae Furu,kawo African Mallow,thutt Leaves, root Diarrhoea, gonorrhoea,antipyretic,cough,piles Ihun,ewon- 4 Acacia ataxacantha Mimosaceae Benin rope Acacia Young leaves Dysentery,backache adele,sarkakiyaa,uke. 5 Acacia auriculiformis Leguminosae Kasia eleti Earleaf acacia Bark Astringent 6 Acacia nilotica Mimosaceae Baani,booni,gabaruwa Acacia,Egyptian mimosa Fruits,bark,exudate Skin diseases,fungal infections,insomnia,emollient. Bark,stem- 7 Acacia sieberiana Mimosaceae Siyi,sie,farakaya Acacia Anti-cancer,antipyretic,astringent,kidney disease,taeniacide twigs,roots,leaves,latex Syphilis, asthma,,anthelmintics, ulcers,rheumatism, antimicrobial 8 Acalypha fimbriata Euphorbiaceae Jinwinini,kandiri Acalypha Leaves & antifungal 9 Acalypha godseffiana Euphorbiaceae Jinwinini Acalypha Leaves, twigs Skin infection, Antimicrobials Cat's tail,chenille 10 Acalypha hispida Euphorbiaceae Jiwene, Jinwinini Leaves, twigs Skin rashes, antimicrobial plant,medusa's locks -

Morphoanatomy and Pharmacognostic Study of the Wood of Croton Echioides, the Northeastern Marapuama
Revista Brasileira de Farmacognosia Brazilian Journal of Pharmacognosy 22(5): 946-956, Sep./Oct. 2012 Morphoanatomy and pharmacognostic study of the wood of Croton echioides, the Northeastern Marapuama Cláudio R. Novello,1 Luís C. Marques,2 Cristine R. Miyazaki,2 Maria A. Milaneze-Gutierre,3 Daniela S. Carneiro-Torres,4 5 *,1 Article Maria H. Sarragiotto, João C. P. de Mello 1Departamento de Farmacia, Universidade Estadual de Maringá, Brazil, 2Universidade Bandeirante de São Paulo, Brazil, 3 Received 23 Jan 2012 Departamento de Biologia, Universidade Estadual de Maringá, Brazil, Accepted 11 Apr 2012 4Universidade Estadual de Feira de Santana, Brazil, Available online 15 May 2012 5Departamento de Química, Universidade Estadual de Maringá, Brazil. Abstract: Croton echioides Baill., Euphorbiaceae, is a small tree found in Bahia, Northeastern Brazil. Its stem bark has been widely sold as an aphrodisiac and Keywords: Croton echioides tonic, as a substitute for the roots of Ptychopetalum olacoides Benth. Olacaceae, Euphorbiaceae the Amazon Muira Puama or Marapuama, and C. echioides is characterized as the morphoanatomy "Northeastern Marapuama". This contribution describes a morphoanatomical analysis Northeastern Marapuama and pharmacognostic study of stem bark of this species. The stem has a thick cortex pharmacognostic quality control with compound starch grains and laticifers; a sclerenchymatic sheath which consists of brachysclereids with large crystals externally to the phloem, and abundant fi ber in the secondary xylem, as the main features of the species. The data obtained for water content (9.26±0.07%), water-soluble extractives (3.92±0.19%), total ash (1.24±0.06%) and acid-insoluble ash (0.16±0.01%), together with the chromatographic profi le obtained by TLC, contribute to the quality control and standardization for the plant drug. -

Antrocom Journal of Anthropology 15-2
Antrocom Journal of Anthropology ANTROCOM journal homepage: http://www.antrocom.net Antrocom Journal of Anthropology 15-2 Antrocom Online Journal of Anthropology vol. 15. n. 2 (2019) – ISSN 1973 – 2880 Periodico on line iscritto nel Registro Stampa presso la Cancelleria del Tribunale di Padova in data 18 Maggio 2017, n° iscrizione 2438 del Registro Stampa Antrocom Online Journal of Anthropology vol. 15. n. 2 (2019) 3-4 – ISSN 1973 – 2880 Summary La plante alan et le culte des ancêtres chez les Fang du Gabon 5 by Giorgio Samorini L’arte rupestre camuna tra Cervi, Caccia Selvaggia, Aquane e Nani minatori 17 by Sandra Busatta Formazione e idealizzazione di tesi assiomatiche in ambito scientifico Il caso di Licini Forum 65 by Fabio Carminati, Andrea Mariani L’accusa di stregoneria nel sistema giudiziario in Repubblica Centrafricana 79 by Chiara Musu Etnografia di un’esperienza in carcere 89 by Claudio Riga Evaluating Factors Affecting Resilience of Natural Resource Dependent Community: A Case of the Tai Khamtis of Arunachal Pradesh, India 109 by Heerock Jyoti Baruah A Study on Dermatoglyphic Patterns Among The Healthy and Hypertensive Bengali Population of Kolkata, West Bengal, India 123 by Titas Ghosh and Monali Goswami Unity and Social Solidarity in a Tribal Village of Dooars 133 by Abhishek Kumar Troubled Mind and Healing Rituals: Re-Thinking Mental Health 141 by Madhur Mrinal Prevalence of hypertension and its concomitants: An exploratory study among a group of bus drivers in Kolkata, West Bengal 149 by Monojit Das, Akash Mallick, Subir Biswas The Determinants of the Fertility Differentials among the Bishnupriyas of Manipur, North East India 159 by Naorem Ranjita 4 Summary / Antrocom Online Journal of Anthropology, vol.