Pollination, Mating System, Phenology and Characterisation of Medinilla Multiflora Merr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogeny and Classification of the Melastomataceae and Memecylaceae
Nord. J. Bot. - Section of tropical taxonomy Phylogeny and classification of the Melastomataceae and Memecy laceae Susanne S. Renner Renner, S. S. 1993. Phylogeny and classification of the Melastomataceae and Memecy- laceae. - Nord. J. Bot. 13: 519-540. Copenhagen. ISSN 0107-055X. A systematic analysis of the Melastomataceae, a pantropical family of about 4200- 4500 species in c. 166 genera, and their traditional allies, the Memecylaceae, with c. 430 species in six genera, suggests a phylogeny in which there are two major lineages in the Melastomataceae and a clearly distinct Memecylaceae. Melastomataceae have close affinities with Crypteroniaceae and Lythraceae, while Memecylaceae seem closer to Myrtaceae, all of which were considered as possible outgroups, but sister group relationships in this plexus could not be resolved. Based on an analysis of all morph- ological and anatomical characters useful for higher level grouping in the Melastoma- taceae and Memecylaceae a cladistic analysis of the evolutionary relationships of the tribes of the Melastomataceae was performed, employing part of the ingroup as outgroup. Using 7 of the 21 characters scored for all genera, the maximum parsimony program PAUP in an exhaustive search found four 8-step trees with a consistency index of 0.86. Because of the limited number of characters used and the uncertain monophyly of some of the tribes, however, all presented phylogenetic hypotheses are weak. A synapomorphy of the Memecylaceae is the presence of a dorsal terpenoid-producing connective gland, a synapomorphy of the Melastomataceae is the perfectly acrodro- mous leaf venation. Within the Melastomataceae, a basal monophyletic group consists of the Kibessioideae (Prernandra) characterized by fiber tracheids, radially and axially included phloem, and median-parietal placentation (placentas along the mid-veins of the locule walls). -

Pertelaan Morfologi Medinilla Spp. Di Kebun Raya “Eka Karya” Bali Dalam Rangka Pengembangan Tanaman Hias
PERTELAAN MORFOLOGI MEDINILLA SPP. DI KEBUN RAYA “EKA KARYA” BALI DALAM RANGKA PENGEMBANGAN TANAMAN HIAS MORPHOLOGICAL DESCRIPTION OF MEDINILLA SPP. IN BALI BOTANIC GARDEN IN ORDER TO DEVELOP AS ORNAMENTAL PLANT I Nyoman Peneng dan Wawan Sujarwo UPT Balai Konservasi Tumbuhan Kebun Raya “Eka Karya” Bali–LIPI Candikuning, Baturiti, Tabanan 82191. Telp. (0368) 2033170, Fax. (0368) 2033171 e-mail: [email protected]; [email protected] ABSTRACT The morphological description of 13 species of Medinilla spp. (Melastomataceae) in Bali Botanic Garden has been conducted based on morphological characters, so that it could be evidence to be justifi ed as ornamental plant. The study was conducted on plant collections of Bali Botanic Garden and the species of Medinilla which is growing wild inside the garden. Morphological data and agronomic potential of each species were showed in order to support its development as ornamental plant. Based on the results of observation of morphological characterization, several species of Medinilla was suitable to be developed as ornamental plant in pots or planted directly in the fi eld. Further explanation of each Medinilla as ornamental plant will be discussed in this paper. Keywords: morphology, Medinilla spp., Bali Botanic Garden, ornamental plant ABSTRAK Pertelaan morfologi dari 13 jenis Medinilla spp. (Melastomataceae) di Kebun Raya Bali telah dilakukan dengan didasarkan pada karakter sifat morfologi, sehingga hal tersebut dapat menjadi bukti untuk menjustifi kasi sebagai tanaman hias. Penelitian dilakukan pada koleksi Medinilla Kebun Raya Bali dan juga spesies Medinilla yang tumbuh liar (pra koleksi) di dalam kebun. Data morfologi dan potensi agronomi dari setiap spesies Medinilla ditunjukkan untuk mendukung pengembangannya sebagai tanaman hias. -

Growing Your Medinilla Speciosa Plant
Growing your Medinilla speciosa plant Medinilla speciosa is a relatively recent introduction to Australia. It is a small to medium shrub, growing to around 2 metres. The large, almost succulent leaves are heavily veined and make an attractive contrast in the garden. Of course the outstanding feature of this plant are the flowers. They are formed in large pendulous racemes. The new flowers open a light pink, followed by hot pink berries which ripen to purple. There is always varying colours of flower and fruit on the shrub at any given time, making it a great specimen for any tropical or sub- tropical garden or even for pot culture. When your plant arrives, it is important to get it potted up as soon as possible. I would use a standard 150-200 mm pot and a well drained soil mix, I use Searle’s brand. Add a teaspoon of Osmocote and water in well. Keep your plant in bright filtered light with maybe some early morning sun for a couple of weeks until it starts to re-establish, then slowly move it to more morning sun with protection from hot afternoon sun. Pot on as needed, the roots are quite fast growing. Occasional trimming after the bulk of the flowering has finished will form a more compact plant. Water well during dry periods and feed once or twice a year with a complete fertiliser or dynamic lifter. The only major pests of this plant are chewing insects such as Grasshoppers and Caterpillars, these can simply be crushed or sprayed with the insecticide Carbaryl. -

Scientia MARDI Food and Agriculture As Well As Poverty African Countries to Overcome Extreme MARDI Headquarters, By: Dario Gilmozzi Reduction
Challenges Innovation As Enabler In In Malaysia’s Developing Agrotourism Agrotourism Industry Products Pg2 Pg3 Apr 2015 Vol: 005 ISSN 2289-6511 9 772289 651006 Malaysia And FAO Strengthen Agrotechnology Parks As MARDI Orchid Hybrids Synchronized Flowering of Tecoma, Partnership Platforms For Agrotourism the Malaysian Cherry Blossoms Pg2 Pg5 Pg6 Pg9 Agrotourism In Malaysia: Direction And Development Strategy Malaysia, contributing 16.1% of the Gross Generally, agrotourism refers to the well as tourism segments. By: Dr. Chubashini Suntharalingam, Domestic Product in 2013 and supporting involvement of agricultural activities for Among the goals of the roadmap is to Rosnani Harun and Nik Rozana Nik Mohd 1.9 million jobs. the purpose of enjoyment and education. make agrotourism as a source of income Masdek, Economic and Technology Between 1998 and 2013, the number of Agrotourism in Malaysia has assisted for local communities. Management Research Centre, MARDI international tourists increased by ap- local communities, such as farmers, Most agrotourism entrepreneurs are proximately five folds, from 5.6 million educate visitors on farming activities and locals in rural communities and this has to 25.7 million. During the same period, in return the farmers are compensated for the potential for increasing the income of income generated by the arrival of in- the services offered. rural communities. The sector is being grotourism is a subsector of the ternational tourists also increased from A good roadmap and clear strategy developed with a variety of products based travel and tourism industry RM8.6 billion to RM65.44 billion. is crucial in any effort to develop on education, agriculture, food, health, that has huge potential and can A Venturing into agrotourism as a di- agrotourism under the National Agrofood homestays, exhibitions, handicrafts, faith increase farmers income regardless of versification strategy has brought great Policy 2011 - 2020. -

Borneo: Sabah
Storm’s Stork (Craig Robson) BORNEO: SABAH 12 – 26 OCTOBER 2019 LEADER CRAIG ROBSON It was back to basics in 2019, with this two-week tour focussing on the Malaysian province of Sabah. Luck was on our side I think, as we succeeded in seeing an excellent range of specialities and endemics. At Kina- balu Park, we found the crucial trio of Whitehead’s Trogon, Whitehead’s Broadbill and Whitehead’s Spider- hunter, as well as the much-wanted Fruithunter - which made an exceptional showing this year -, and Moun- tain Blackeye. At Sepilok and Gomantong we added Bat Hawk and Black-crowned Pitta, while the Kina- batangan River brought us the likes of Storm’s Stork, Bornean Ground Cuckoo, Wrinkled Hornbill, and Hooded Pitta. Our final destination at the luxurious Borneo Rainforest Lodge, in Danum Valley Conservation Area, produced the bird of the tour - Bornean Bristlehead - as well as ‘Bornean’ Crested Fireback, Barred 1 BirdQuest Tour Report: Borneo: Sabah www.birdquest-tours.com Eagle-Owl, Large, Gould’s and Sunda Frogmouths, Blue-headed and Bornean Banded Pittas, and Bornean and Black-throated Wren-Babblers. Mammals featured prominently too with several Bornean Orangutans, hundreds of Proboscis Monkeys, and several fantastic Horsfield’s Tarsiers to name but a few. There was also a wide range of other interesting wildlife, from reptiles like Bornean Keeled Pit Viper, to spectacular butterflies like Rajah Brooke’s Birdwing. Having all met up at the airport in Kota Kinabalu, around midday, we headed off birding for the rest of the day. First up was Lok Kawi Beach, where we observed a range of shorebirds, including a dapper pair of Malaysian Plovers, and common migrant species such as Terek Sandpiper and Grey-tailed Tattler. -
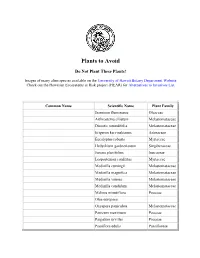
Plants to Avoid
Plants to Avoid Do ot Plant These Plants! Images of many alien species available on the University of Hawaii Botany Department Website Check out the Hawaiian Ecosystems at Risk project (HEAR) for Alternatives to Invasives List Common ame Scientific ame Plant Family Jasminim fluminense Oleaceae Arthrostema ciliatum Melastomataceae Dissotis rotundifolia Melastomataceae Erigeron karvinskianus Asteraceae Eucalyptus robusta Myrtaceae Hedychium gardnerianum Singiberaceae Juncus planifolius Juncaceae Lospostemon confertus Myrtaceae Medinilla cumingii Melastomataceae Medinilla magnifica Melastomataceae Medinilla venosa Melastomataceae Medinilla candidum Melastomataceae Melinis minutiflora Poaceae Olea europaea Oxyspora paniculata Melastomataceae Panicum maximum Poaceae Paspalum urvillei Poaceae Passiflora edulis Passifloreae Phormium tenax Agavaceae Pinus taeda Pinaceae Prosopis pallida Favaceae Pterolepis glomerata Melastomataceae Rhodomyrtus tomentosa Myrtaceae Schefflera actinophylla Araliaceae Syzygium jambos Myrtaceae Australian blackwood Acacia melanoxylon Mimosaceae Australian tree fern Cyathea cooperi Cyatheaceae Australian tree fern Sphaeropteris cooperi Cyatheaceae Beggar's tick, Spanish needle Bidens pilosa Asteraceae California grass Brachiaria mutica Poaceae Chinese banyan, Malayan banyan Ficus mirocarpa Moraceae Chinese violet Asystasia gangetica Acanthaceae Christmasberry, Brazilian pepper Schinus terebinthifolius Anacardiaceae Formosan koa Acacia confusa Mimosaceae German ivy Senecio mikanioides Asteraceae Japanese honeysuckle -

Melastomataceae) of Borneo
BLUMEA 35 (1990) 5-70 Revision of Medinilla(Melastomataceae) of Borneo Jacinto+C. Regalado+Jr. Department of Botany and Plant Pathology, Michigan State University, East Lansing, Michigan 48824,U. S. A.* Summary Forty-eight species ofMedinilla are now known from Borneo, 28 of which are describedas new. At least 20 taxa are known only from one to three collections. Eleven species groups have been of the awaits further of recognized and defined.A more thorough understanding genus study Philip- pine and New Guinea materials. A key to the Bornean species, illustrations of 15 species, and eco- logical notes are provided. Two previously described species are recorded for the first time for Bor- neo: Medinilla succulenta (Blume) Blume, and M. pterocaula Blume. One new combinationand five reductions have been made. Medinilla tawaensis Merrill is transferred to Catanthera; M. caudatifolia Schwartz and M. hasseltii Blume var. subsessilis Schwartz are reduced to M. crassifolia (Reinw. ex Blume) Blume; M. dajakorum Schwartz is reduced to M. corallina Cogn.; M. borneensis Blume and M. motleyi Hook. f. ex Triana are conspecific with M. macrophylla Blume. Introduction Medinilla (Melastomataceae) is a genus of epiphytic and terrestrial shrubs and climbersof the Paleotropics. It includes about 400 species (Shaw, 1973) distributed in Africa, Madagascar, India, Ceylon, Burma, Indochina,southern China, Thailand, the Malay Peninsula, and eastward to the islands of the Malay Archipelago, New Guinea, northern Australia, Micronesia, and Melanesia. It is by far the largest of all melastome genera occurring in Malesia, a floristic region made up of the Malay Peninsula and islands of the Malay Archipelago extending to New Guinea. -

Seminar Nasional&
Seminar Nasional& International Conference Pros Sem Nas Masy Biodiv Indon vol. 3 | no. 3 | pp. 287‐475| Desember 2017 ISSN: 2407‐8050 wisatajawa.co.id foto: , Barat Penyelenggara & Jawa Pendukung Remis, Telaga | vol. 3 | no. 3 | pp. 287-475 | Desember 2017 | ISSN: 2407-8050 | DEWAN PENYUNTING: Ketua, Ahmad Dwi Setyawan, Universitas Sebelas Maret, Surakarta Anggota, Sugiyarto, Universitas Sebelas Maret, Surakarta Anggota, Ari Pitoyo, Universitas Sebelas Maret, Surakarta Anggota, Sutomo, UPT Balai Konservasi Tumbuhan Kebun Raya “Eka Karya”, LIPI, Tabanan, Bali Anggota, A. Widiastuti, Balai Besar Pengembangan Pengujian Mutu Benih Tanaman Pangan dan Hortikultura, Depok Anggota, Gut Windarsih, IAIN Sultan Maulana Hasanuddin, Serang Anggota, Supatmi, Pusat Penelitian Bioteknologi, LIPI, Cibinong, Bogor PENYUNTING TAMU (PENASEHAT): Abinawanto, Universitas Indonesia, Jakarta Mustaid Siregar, PKT Kebun Raya Indonesia, Bogor Ruhyat Partasasmita, Universitas Padjadjaran, Sumedang PENERBIT: Masyarakat Biodiversitas Indonesia PENERBIT PENDAMPING: Program Biosains, Program Pascasarjana, Universitas Sebelas Maret Surakarta Jurusan Biologi, FMIPA, Universitas Sebelas Maret Surakarta PUBLIKASI PERDANA: 2015 ALAMAT: Kantor Jurnal Biodiversitas, Jurusan Biologi, FMIPA, Universitas Sebelas Maret Jl. Ir. Sutami 36A Surakarta 57126. Tel. & Fax.: +62-271-663375, Email: [email protected] ONLINE: biodiversitas.mipa.uns.ac.id/psnmbi.htm PENYELENGGARA & PENDUKUNG: MASYARAKAT BIODIVERSITAS INDONESIA JUR. BIOLOGI FMIPA & FAKULTAS KEHUTANAN PKT KEBUN RAYA DEP. BIOLOGI FMIPA PS. BIOSAINS PPS IPB BOGOR INDONESIA UI DEPOK UNS SURAKARTA (KEBUN RAYA BOGOR) THIS PAGE INTENTIONALLY LEFT BLANK Pedoman untuk Penulis Ruang Lingkup Prosiding Seminar Nasional Masyarakat Biodiversitas al. 2008). Kutipan bertingkat seperti yang ditunjukkan dengan kata cit. Indonesia (Pros Sem Nas Masy Biodiv Indon) menerbitkan naskah atau dalam harus dihindari. bertemakan keanekaragaman hayati pada tumbuhan, hewan dan mikroba, pada tingkat gen, spesies dan ekosistem serta etnobiologi (pemanfaatan). -

Lampiran Surat : Nomor : 117/B3.1/Km/2016 Tanggal : 19 Februari 2016
LAMPIRAN SURAT : NOMOR : 117/B3.1/KM/2016 TANGGAL : 19 FEBRUARI 2016 DAFTAR PENERIMA HIBAH PROGRAM KREATIVITAS MAHASISWA (PKM) UNTUK PERGURUAN TINGGI NEGERI TAHUN 2016 NO. NAMA PELAKSANA JUDUL KEGIATAN PERGURUAN TINGGI SKEMA 1 Febry Crunchy On Chocolate : Camilan Cokelat dalam Jar Institut Pertanian Bogor PKMK Nata de Taro - Pemanfaatan Limbah Cair Tepung Talas 2 Fatimatus Zuhro Institut Pertanian Bogor PKMK sebagai Oleh-Oleh Khas Bogor pluma (painted feather accesories) inovasi bisnis bulu 3 Fathya Fiddini Elfajri Institut Pertanian Bogor PKMK unggas handcraft KROTO BESEK: BUDIDAYA KROTO DENGAN SISTEM 4 Tatang Dwi Utomo BESEK SOLUSI MENGHASILKAN SIKLUS PRODUKSI Institut Pertanian Bogor PKMK KROTO YANG CEPAT "Del'covy" Delicious Anchovy: Ikan Teri Medan Ready 5 Hafizh Abdul Aziz Institut Pertanian Bogor PKMK to Eat dengan Varian Rasa Gurinori Snack Daun Singkong: Produk Nori 6 Hayah Afifah Termodifikasi Pangan Lokal Bergizi Institut Pertanian Bogor PKMK O.. I SEE sebagai Sarana Meningkatkan Pengetahuan 7 Rose Anwarni Institut Pertanian Bogor PKMK tentang Laut untuk Dunia International "SARI PERFUME": PARFUM ISLAMI BERBAHAN Muhammad Fikri Abdul 8 MINYAK ATSIRI LOKAL SEBAGAI PRODUK UNGGULAN Institut Pertanian Bogor PKMK Alim INDONESIA ROK TU-IN-ROK SEBAGAI INOVASI USAHA DENGAN 9 Priyanti DESAIN ROK YANG DIGUNAKAN BOLAK-BALIK Institut Pertanian Bogor PKMK MELALUI PEMANFAATAN KAIN PRODUK LOKAL TEH KUKU IPB INOVASI TEH TERBARU BERBAHAN 10 Abdul Salam DASAR KUMIS KUCING DENGAN PEMANIS ALAMI Institut Pertanian Bogor PKMK RENDAH KALORI "WALL-MUSH" Hiasan Edukatif Pertanian Berbasis 11 Lutfi Ilham Pradipta Institut Pertanian Bogor PKMK Budidaya Jamur Agripedia : Usaha Pertanian Berbasis Syariah Sebagai 12 David Anwar Institut Pertanian Bogor PKMK Wadah Inovasi Pertanian yang Solutif dan Inovatif PORL Pomade Rumput Laut Sargassum sp. -

Structural Basis for Divergent and Convergent Evolution of Catalytic Machineries in Plant Aromatic Amino Acid Decarboxylase Proteins
Structural basis for divergent and convergent evolution of catalytic machineries in plant aromatic amino acid decarboxylase proteins Michael P. Torrens-Spencea, Ying-Chih Chiangb,1, Tyler Smitha,c, Maria A. Vicenta,d, Yi Wangb, and Jing-Ke Wenga,c,2 aWhitehead Institute for Biomedical Research, Cambridge, MA 02142; bDepartment of Physics, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong; cDepartment of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139; and dDepartment of Biology, Williams College, Williamstown, MA 01267 Edited by Richard A. Dixon, University of North Texas, Denton, TX, and approved March 28, 2020 (received for review November 14, 2019) Radiation of the plant pyridoxal 5′-phosphate (PLP)-dependent aro- such as dopamine and serotonin, from their respective aromatic matic L-amino acid decarboxylase (AAAD) family has yielded an ar- L-amino acid precursors (5). AAAD family enzymes have radi- ray of paralogous enzymes exhibiting divergent substrate preferences ated in plants and insects to yield a number of paralogous en- and catalytic mechanisms. Plant AAADs catalyze either the decar- zymes with variation in both substrate preference and catalytic boxylation or decarboxylation-dependent oxidative deamination mechanism (Fig. 1 A–C and SI Appendix, Fig. S1) (6). In plants, of aromatic L-amino acids to produce aromatic monoamines or L-tryptophan decarboxylases (TDCs) and L-tyrosine decarbox- aromatic acetaldehydes, respectively. These compounds serve as ylases (TyDCs) are canonical AAADs that supply the aromatic key precursors for the biosynthesis of several important classes of monoamine precursors tryptamine and tyramine for the bio- plant natural products, including indole alkaloids, benzylisoquino- line alkaloids, hydroxycinnamic acid amides, phenylacetaldehyde- synthesis of monoterpene indole alkaloids (MIAs) and benzyli- derived floral volatiles, and tyrosol derivatives. -

Ornamental Flowers, Buds and Leaves Pdf, Epub, Ebook
ORNAMENTAL FLOWERS, BUDS AND LEAVES PDF, EPUB, EBOOK V. Ruprich-robert | 144 pages | 28 Jan 2011 | Dover Publications Inc. | 9780486477749 | English | New York, United States Ornamental Flowers, Buds and Leaves PDF Book All plant parts of oleander are toxic, which, when ingested, can cause severe health conditions both in people and animals. Dicentra 'Gold Heart'. Medinilla magnifica is also known as rose grape or Showy Medinilla and originated in the Philippines. The plant establishes quickly and blooms throughout the summer months. Stays compact, has stronger stems, with a more upright habit than most older varieties. Hosta 'Afterglow'. Allan Armitage for its heat and humidity tolerance. Because of the similarity, tulip bulbs are unintentionally consumed mostly by animals like dogs. Nice in containers. Dicentra 'Luxuriant'. They may look alluring and harmless, but appearances can be deceiving. Flashy green and yellow variegated foliage takes on pink and orange tones in cool weather. This semi-running woodland perennial has bright green foliage in spring and is deeply lobed with pointy edges and an overall lacy texture. It is prevalent during damp, rainy weather. Grows quickly to form a large clump of rounded, bright gold leaves with very wide, dark green margins. From a distance, the flowers look completely white. Lantana flowers attract butterflies and bees with their fragrance, but beyond all that is a potential danger that could do more harm than good to humans and animals. Planting Peruvian lilies as houseplants require extra caution since they are highly poisonous. Wonderful food source for bees in early Spring. The tulip bulbs have the highest concentration of this allergen. -

9 Costion Plant Endemism 133-166 PROOFS
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/265109793 Plant Endemism, Rarity, and Threat in Palau, Micronesia: A Geographical Checklist and Preliminary Red List Assessment Article in Micronesica · January 2009 CITATIONS READS 8 178 3 authors, including: Craig Costion Ann Kitalong Smithsonian Institution Independent Researcher 38 PUBLICATIONS 263 CITATIONS 18 PUBLICATIONS 52 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: The Environment, Inc. and Belau National Museum Herbarium View project The Environment Inc View project All content following this page was uploaded by Craig Costion on 24 November 2014. The user has requested enhancement of the downloaded file. Micronesica 41(1): 131–164, 2009 Plant Endemism, Rarity, and Threat in Palau, Micronesia: A Geographical Checklist and Preliminary Red List Assessment 1 CRAIG M. COSTION Department of Ecology and Evolutionary Biology, School of Earth and Environmental Sciences, University of Adelaide, Adelaide SA 5001 [email protected] ANN HILLMANN KITALONG The Environment, Inc., P.O. Box 1696, Koror, Palau 96940 TARITA HOLM Palau Conservation Society/PALARIS, P.O. Box 1811, Koror, Palau, 96940 Abstract—An official checklist of the endemic plant species of Palau has been long awaited, and is presented here for the first time. For each species a substrate limitation, growth form, and relative abundance is listed. In addition an IUCN red list assessment was conducted using all available data. For over half of the endemic species there is insufficient data to provide a red listing status however an expected minimum number of threatened plants out of the total is inferred.