Correlations Among Fruit Traits and Evolution of Different Fruits Within
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proposal for the Development of Large Scale Seed Production and Roadside Establishment Protocol for Five Native Hawaiian Groundcovers
TERMINATION REPORT FOR (TA) DL2012-2 Proposal for the Development of Large Scale Seed Production and Roadside Establishment Protocol for Five Native Hawaiian Groundcovers. PREPARED BY Dr. Joseph DeFrank, project PI DATED: July 05, 2018 TERINATION REPORT FOR - (TA) DL 2012-2 - July 05, 2018 Page 1 Table of Contents Page Description number Executive Summary of Project Accomplishments 2-3 Establishing seed production nursery on Oahu. 4-10 Weed control research with native plants. 11-16 Seed Harvest Index for Aalii (Dodonaea viscosa) 17-19 Seed Harvest Index for Ahinahina (Achyranthes splendens) 19-23 Seed Harvest Index for Aweoweo (Chenopodium oahuense) 24-25 Seed Harvest Index for Ilima (Sida fallex) 26-27 Seed Harvest Index for Uhaloa (Waltheria indica) 28-30 Executive Summary of Project Accomplishments The Hawaii Department of Transportation has provided funding in support of the research and development project titled: “Proposal for the Development of Large Scale Seed Production and Roadside Establishment Protocol for Five Native Hawaiian Groundcovers”. The notice to proceed date was May15, 2015 with termination date of May 15, 2018. The Task Agreement (TA) for this project is DL2012-2 with Purchase Order No. 40055133. The Cooperative Agreement number is DOT-10-030. Summary of work performed during the project period Establishing seed production nurseries on Oahu. A .9 acre seed production nursery was established in the median area on the leeward side of Oahu in the Halawa interchange, see photos 1-7. All five of the project native plant species are included in this nursery. The nursery is supplied with automatic irrigation. Water conservation and clean seed collection is enhanced due to the used of durable woven black plastic ground cover used extensively throughout the planting. -

Flowering Plant Families of Northwestern California: a Tabular Comparison
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 12-2019 Flowering Plant Families of Northwestern California: A Tabular Comparison James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Flowering Plant Families of Northwestern California: A Tabular Comparison" (2019). Botanical Studies. 95. https://digitalcommons.humboldt.edu/botany_jps/95 This Flora of Northwest California-Regional is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. FLOWERING PLANT FAMILIES OF NORTHWESTERN CALIFORNIA: A TABULAR COMPARISON James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University December 2019 Scientific Name Habit Leaves Sexuality • Floral Formula Common Name Fruit Type • Comments Aceraceae TSV SC:O U-m [P] • K 4-5 C 4-5 A 4-10 G (2) Maple Paired samaras • leaves often palmately lobed Acoraceae H S:A U-m • P 3+3 A 6 or G (3) Sweet Flag Berry • aquatic; aromatic rhizomes Aizoaceae HS S:AO B • P [3] 5 [8] A 0-4 Gsi (2-5-4) Ice Plant Capsule (berry-like) • fleshy; stamens divided, petaloid Alismataceae -

Survey of Roadside Alien Plants in Hawai`I Volcanoes National Park and Adjacent Residential Areas 2001–2005
Technical Report HCSU-032 SURVEY OF ROADSIDE ALIEN PLANts IN HAWAI`I VOLCANOES NATIONAL PARK AND ADJACENT RESIDENTIAL AREAS 2001–2005 Linda W. Pratt1 Keali`i F. Bio2 James D. Jacobi1 1 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Kilauea Field Station, P.O. Box 44, Hawaii National Park, HI 96718 2 Hawai‘i Cooperative Studies Unit, University of Hawai‘i at Hilo, P.O. Box 44, Hawai‘i National Park, HI 96718 Hawai‘i Cooperative Studies Unit University of Hawai‘i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 September 2012 This product was prepared under Cooperative Agreement CA03WRAG0036 for the Pacific Island Ecosystems Research Center of the U.S. Geological Survey. Technical Report HCSU-032 SURVEY OF ROADSIDE ALIEN PLANTS IN HAWAI`I VOLCANOES NATIONAL PARK AND ADJACENT RESIDENTIAL AREAS 2001–2005 1 2 1 LINDA W. PRATT , KEALI`I F. BIO , AND JAMES D. JACOBI 1 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Kīlauea Field Station, P.O. Box 44, Hawai`i Volcanoes National Park, HI 96718 2 Hawaii Cooperative Studies Unit, University of Hawai`i at Hilo, Hilo, HI 96720 Hawai`i Cooperative Studies Unit University of Hawai`i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 September 2012 This article has been peer reviewed and approved for publication consistent with USGS Fundamental Science Practices ( http://pubs.usgs.gov/circ/1367/ ). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. -

Fruits: Kinds and Terms
FRUITS: KINDS AND TERMS THE IMPORTANT PART OF THE LIFE CYCLE OFTEN IGNORED Technically, fruits are the mature ovaries of plants that contain ripe seeds ready for dispersal • Of the many kinds of fruits, there are three basic categories: • Dehiscent fruits that split open to shed their seeds, • Indehiscent dry fruits that retain their seeds and are often dispersed as though they were the seed, and • Indehiscent fleshy fruits that turn color and entice animals to eat them, meanwhile allowing the undigested seeds to pass from the animal’s gut We’ll start with dehiscent fruits. The most basic kind, the follicle, contains a single chamber and opens by one lengthwise slit. The columbine seed pods, three per flower, are follicles A mature columbine follicle Milkweed seed pods are also large follicles. Here the follicle hasn’t yet opened. Here is the milkweed follicle opened The legume is a similar seed pod except it opens by two longitudinal slits, one on either side of the fruit. Here you see seeds displayed from a typical legume. Legumes are only found in the pea family Fabaceae. On this fairy duster legume, you can see the two borders that will later split open. Redbud legumes are colorful before they dry and open Lupine legumes twist as they open, projecting the seeds away from the parent The bur clover modifies its legumes by coiling them and providing them with hooked barbs, only opening later as they dry out. The rattlepods or astragaluses modify their legumes by inflating them for wind dispersal, later opening to shed their seeds. -

Vitales, C Nymphaeales Austrobaileyales
Amborellales Vitales, C Nymphaeales Austrobaileyales Acorales G Eenzaadlobbigen G Alismatales Vitales Petrosaviales Pandanales De Wijnstokfamilie (Vitaceae), d Dioscoreales vroeger samen met de Wegedoo Liliales geplaatst, omdat in beide famil Asparagales kroonbladen staan. Dat de Vital Arecales maar niet precies waar. Hiervoo G Commeliniden G Dasypogonales Poales Commelinales Crossosomatales Zingiberales Deze nieuwe orde in de Rosiden ordes en wordt ondersteund do Ceratophyllales kenmerken, zoals de structuur v Chloranthales afkomstig uit de Violales, Celast Het zijn 5 kleine families uit wa Canellales Piperales Aphloiaceae, Geissolomataceae G Magnoliiden G Magnoliales Stachyuraceae, en 2 iets grotere Laurales (Staphyleacea) en de Crossosom Ranunculales Sabiales Proteales Vitaceae Trochodendrales Buxales Aphloiacea Geissoloma Gunnerales Ixerbaceae Berberidopsidales Strasburge Dilleniales Staphyleac Caryophyllales Stachyurac Santalales Crossosom Saxifragales Melianthac G Geavanceerde tweezaadlobbigen G Vitales Francoacea Crossosomatales Ledocarpa Geraniales Vivianiacea Myrtales Geraniacea Zygophyllales Combretac Celastrales Lythraceae Malpighiales Onagracea G Fabiden G Oxalidales Vochysiace Fabales Myrtaceae G Rosiden G Rosales Crypteroni Cucurbitales Alzateacea Fagales Rhynchoca Oliniaceae Brassicales Penaeacea G G Malviden Malvales Melastoma Sapindales Cornales kenmerken. Uit de Polygalales z Ericales G Asteriden G erbij gevoegd, een kleine famili Garryales Afrika en Amerika. G Lamiiden G Gentianales De bladeren zijn meestal tegeno Solanales -

Systematics and Relationships of Tryssophyton (Melastomataceae
A peer-reviewed open-access journal PhytoKeys 136: 1–21 (2019)Systematics and relationships of Tryssophyton (Melastomataceae) 1 doi: 10.3897/phytokeys.136.38558 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research Systematics and relationships of Tryssophyton (Melastomataceae), with a second species from the Pakaraima Mountains of Guyana Kenneth J. Wurdack1, Fabián A. Michelangeli2 1 Department of Botany, MRC-166 National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, Washington, DC 20013-7012, USA 2 The New York Botanical Garden, 2900 Southern Blvd., Bronx, NY 10458, USA Corresponding author: Kenneth J. Wurdack ([email protected]) Academic editor: Ricardo Kriebel | Received 25 July 2019 | Accepted 30 October 2019 | Published 10 December 2019 Citation: Wurdack KJ, Michelangeli FA (2019) Systematics and relationships of Tryssophyton (Melastomataceae), with a second species from the Pakaraima Mountains of Guyana. PhytoKeys 136: 1–21. https://doi.org/10.3897/ phytokeys.136.38558 Abstract The systematics of Tryssophyton, herbs endemic to the Pakaraima Mountains of western Guyana, is re- viewed and Tryssophyton quadrifolius K.Wurdack & Michelang., sp. nov. from the summit of Kamakusa Mountain is described as the second species in the genus. The new species is distinguished from its closest relative, Tryssophyton merumense, by striking vegetative differences, including number of leaves per stem and leaf architecture. A phylogenetic analysis of sequence data from three plastid loci and Melastomata- ceae-wide taxon sampling is presented. The two species of Tryssophyton are recovered as monophyletic and associated with mostly Old World tribe Sonerileae. Fruit, seed and leaf morphology are described for the first time, biogeography is discussed and both species are illustrated. -

The Uses of Molecular Dating Analyses in Evolutionary Studies, with Examples from the Angiosperms
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2006 The uses of molecular dating analyses in evolutionary studies, with examples from the angiosperms Rutschmann, Frank Kaspar Abstract: Die sechs Kapitel der vorliegenden Dissertation befassen sich alle mit verschiedenen Aspekten von molekularen Datierungsmethoden, englisch molecular dating methods genannt. Dabei reicht die Spannweite der behandelten Themen von experimentellen Untersuchungen der Methoden selbst bis hin zur praktischen Anwendung der molekularen Altersbestimmung für die Aufklärung von biologischen und evolutionsgeschichtlichen Fragen bei verschiedenen Gruppen von Blütenpflanzen (Angiospermen). The six chapters that compose this dissertation are all related to various aspects of molecular dating, ranging from more methodological and experimental work to the application of different methods in the context of biological and evolutionary questions and hypotheses related to different groups of flowering plants (Angiosperms). Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-163459 Dissertation Published Version Originally published at: Rutschmann, Frank Kaspar. The uses of molecular dating analyses in evolutionary studies, with examples from the angiosperms. 2006, University of Zurich, Faculty of Science. The Uses of Molecular Dating Analyses in Evolutionary Studies, with Examples from the Angiosperms Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr. sc. nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultt der niversitt Zürich von Frank Kaspar Rutschmann von Zürich ZH Promotionskomitee: Prof. Dr. Elena Conti ()orsitz) Prof. Dr. Peter Linder Dr. Torsten Eriksson Zürich 200. Acknowledgements First of all, I would like to express my gratitude towards my supervisor, Elena Conti. -

Additions to the Flora of Panama, with Comments on Plant Collections and Information Gaps
15 4 NOTES ON GEOGRAPHIC DISTRIBUTION Check List 15 (4): 601–627 https://doi.org/10.15560/15.4.601 Additions to the flora of Panama, with comments on plant collections and information gaps Orlando O. Ortiz1, Rodolfo Flores2, Gordon McPherson3, Juan F. Carrión4, Ernesto Campos-Pineda5, Riccardo M. Baldini6 1 Herbario PMA, Universidad de Panamá, Vía Simón Bolívar, Panama City, Panama Province, Estafeta Universitaria, Panama. 2 Programa de Maestría en Biología Vegetal, Universidad Autónoma de Chiriquí, El Cabrero, David City, Chiriquí Province, Panama. 3 Herbarium, Missouri Botanical Garden, 4500 Shaw Boulevard, St. Louis, Missouri, MO 63166-0299, USA. 4 Programa de Pós-Graduação em Botânica, Universidade Estadual de Feira de Santana, Avenida Transnordestina s/n, Novo Horizonte, 44036-900, Feira de Santana, Bahia, Brazil. 5 Smithsonian Tropical Research Institute, Luis Clement Avenue (Ancón, Tupper 401), Panama City, Panama Province, Panama. 6 Centro Studi Erbario Tropicale (FT herbarium) and Dipartimento di Biologia, Università di Firenze, Via La Pira 4, 50121, Firenze, Italy. Corresponding author: Orlando O. Ortiz, [email protected]. Abstract In the present study, we report 46 new records of vascular plants species from Panama. The species belong to the fol- lowing families: Anacardiaceae, Apocynaceae, Aquifoliaceae, Araceae, Bignoniaceae, Burseraceae, Caryocaraceae, Celastraceae, Chrysobalanaceae, Cucurbitaceae, Erythroxylaceae, Euphorbiaceae, Fabaceae, Gentianaceae, Laciste- mataceae, Lauraceae, Malpighiaceae, Malvaceae, Marattiaceae, Melastomataceae, Moraceae, Myrtaceae, Ochnaceae, Orchidaceae, Passifloraceae, Peraceae, Poaceae, Portulacaceae, Ranunculaceae, Salicaceae, Sapindaceae, Sapotaceae, Solanaceae, and Violaceae. Additionally, the status of plant collections in Panama is discussed; we focused on the areas where we identified significant information gaps regarding real assessments of plant biodiversity in the country. -

Liber Amicorum Prof. Dr. Ir. R.A.A. Oldeman
25 Jaar een ”Boom der vrijheid” Liber Amicorum Prof Dr Ir R.A.A. Oldeman Edited by Hans Vester, Paul Romeijn and Hans van der Wal Treemail publishers Heelsum, The Netherlands Treebook 8 October 2004, ISBN 90-804443-9-1 Treemail Publishers, Heelsum, The Netherlands Earlier in the series: Treebook 1: Struggle of Life: or the natural history of stress and adaptation. Martial and Line Rossignol, Roelof A. A. Oldeman and Soraya Benzine-Tizroutine; 1998. Treebook 2: Green Gold: on variations of truth in plantation forestry. Paul Romeijn; 1999. Treebook 3: Chinantec shifting cultivation: InTERAcTIVE landuse. A case-study in the Chinantla, Mexico, on secondary vegetation, soils and crop performance under indigenous shifting cultivation. Hans van der Wal; 1999. Treebook 4: An exploratory study to improve the predictive capacity of the Crop Growth Monitoring System as applied by the European Commission. Iwan Supit; 2000. Treebook 5: Five thousand years of sustainability? A case study on Gedeo land use (Southern Ethiopia). Tadesse Kippie; 2002. Treebook 6: Let them eat grass: Understanding pasture-finished beef cattle farms in the American Appalachians. Jane Shaw; 2002. Treebook 7: Updated system description of the WOFOST crop growth simulation model as implemented in the Crop Growth Monitoring System applied by the European Commission. Supit, I.; Van der Goot, E.; Editors; January 2003. © 2004, Copyright by Treemail. All rights reserved. No part of these materials may be reproduced, or stored in a retrieval system, or transmitted, in any form, or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the copyright owner. -

Trait Correlates and Functional Significance of Heteranthery In
New Research Phytologist Trait correlates and functional significance of heteranthery in flowering plants Mario Vallejo-Marı´n1, Elizabeth M. Da Silva2, Risa D. Sargent2 and Spencer C. H. Barrett3 1School of Biological and Environmental Sciences, University of Stirling, Stirling FK9 4LA, UK; 2Department of Biology, University of Ottawa, 30 Marie-Curie (160 Gendron), Ottawa, ON K1N 6N5, Canada; 3Department of Ecology and Evolutionary Biology, University of Toronto, 25 Willcocks Street, Toronto, ON M5S 3B2, Canada Summary Author for correspondence: • Flowering plants display extraordinary diversity in the morphology of male sexual Mario Vallejo-Marı´n organs, yet the functional significance of this variation is not well understood. Tel: +44 1786 467822 Here, we conducted a comparative analysis of floral correlates of heteranthery – Email: [email protected] the morphological and functional differentiation of anthers within flowers – among Received: 25 June 2010 angiosperm families to identify traits associated with this condition. Accepted: 15 July 2010 • We performed a phylogenetic analysis of correlated evolution between hete- ranthery and several floral traits commonly reported from heterantherous taxa. In New Phytologist (2010) 188: 418–425 addition, we quantified the effect of phylogenetic uncertainty in the observed pat- doi: 10.1111/j.1469-8137.2010.03430.x terns of correlated evolution by comparing trees in which polytomous branches were randomly resolved. • Heteranthery is reported from 12 angiosperm orders and is phylogenetically Key words: buzz-pollination, division of labour, heteranthery, phylogenetic analysis, associated with the absence of floral nectaries, buzz-pollination and enantiostyly stamen differentiation. (mirror-image flowers). These associations are robust to particularities of the underlying phylogenetic hypothesis. -
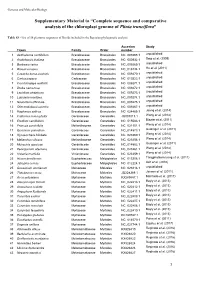
Complete Sequence and Comparative Analysis of the Chloroplast Genome of Plinia Trunciflora”
Genetics and Molecular Biology Supplementary Material to “Complete sequence and comparative analysis of the chloroplast genome of Plinia trunciflora” Table S3 - List of 56 plastome sequences of Rosids included in the Bayesian phylogenetic analysis. Accesion Study Taxon Family Order number 1 Aethionema cordifolium Brassicaceae Brassicales NC_009265.1 unpublished 2 Arabidopsis thaliana Brassicaceae Brassicales NC_000932.1 Sato et al. (1999) 3 Barbarea verna Brassicaceae Brassicales NC_009269.1 unpublished 4 Brassica napus Brassicaceae Brassicales NC_016734.1 Hu et al. (2011) 5 Capsella bursa-pastoris Brassicaceae Brassicales NC_009270.1 unpublished 6 Carica papaya Caricaceae Brassicales NC_010323.1 unpublished 7 Crucihimalaya wallichii Brassicaceae Brassicales NC_009271.1 unpublished 8 Draba nemorosa Brassicaceae Brassicales NC_009272.1 unpublished 9 Lepidium virginicum Brassicaceae Brassicales NC_009273.1 unpublished 10 Lobularia maritima Brassicaceae Brassicales NC_009274.1 unpublished 11 Nasturtium officinale Brassicaceae Brassicales NC_009275.1 unpublished 12 Olimarabidopsis pumila Brassicaceae Brassicales NC_009267.1 unpublished 13 Raphanus sativus Brassicaceae Brassicales NC_024469.1 Jeong et al. (2014) 14 California macrophylla Geraniaceae Geraniales JQ031013.1 Weng et al. (2014) 15 Erodium carvifolium Geraniaceae Geraniales NC_015083.1 Blazier et al. (2011) 16 Francoa sonchifolia Melianthaceae Geraniales NC_021101.1 Weng et al. (2014) 17 Geranium palmatum Geraniaceae Geraniales NC_014573.1 Guisinger et al. (2011) 18 Hypseocharis bilobate Geraniaceae Geraniales NC_023260.1 Weng et al. (2014) 19 Melianthus villosus Melianthaceae Geraniales NC_023256.1 Weng et al. (2014) 20 Monsonia speciose Geraniaceae Geraniales NC_014582.1 Guisinger et al. (2011) 21 Pelargonium alternans Geraniaceae Geraniales NC_023261.1 Weng et al. (2014) 22 Viviania marifolia Vivianiaceae Geraniales NC_023259.1 Weng et al. (2014) 23 Hevea brasiliensis Euphorbiaceae Malpighiales NC_015308.1 Tangphatsornruang et al. -

Molecular Phylogenetics of Melastomataceae and Memecylaceae: Implications for Character Evolution1
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Open Access LMU American Journal of Botany 88(3): 486±498. 2001. MOLECULAR PHYLOGENETICS OF MELASTOMATACEAE AND MEMECYLACEAE: IMPLICATIONS FOR CHARACTER EVOLUTION1 G. CLAUSING2,4 AND S. S. RENNER3,4 2Institut fuÈr Spezielle Botanik, UniversitaÈt Mainz, D-55099 Mainz, Germany; 3Department of Biology, University of Missouri-St. Louis, 8001 Natural Bridge Rd., St. Louis, Missouri 63121 USA; and The Missouri Botanical Garden, St. Louis, Missouri 63166 USA Melastomataceae are among the most abundant and diversi®ed groups of plants throughout the tropics, but their intrafamily rela- tionships and morphological evolution are poorly understood. Here we report the results of parsimony and maximum likelihood (ML) analyses of cpDNA sequences from the rbcL and ndhF genes and the rpl16 intron, generated for eight outgroups (Crypteroniaceae, Alzateaceae, Rhynchocalycaceae, Oliniaceae, Penaeaceae, Myrtaceae, and Onagraceae) and 54 species of melastomes. The sample represents 42 of the family's currently recognized ;150 genera, the 13 traditional tribes, and the three subfamilies, Astronioideae, Melastomatoideae, and Memecyloideae (5 Memecylaceae DC.). Parsimony and ML yield congruent topologies that place Memecy- laceae as sister to Melastomataceae. Pternandra, a Southeast Asian genus of 15 species of which ®ve were sampled, is the ®rst- branching Melastomataceae. This placement has low bootstrap support (72%), but agrees with morphological treatments that placed Pternandra in Melastomatacaeae because of its acrodromal leaf venation, usually ranked as a tribe or subfamily. The interxylary phloem islands found in Memecylaceae and Pternandra, but not most other Melastomataceae, likely evolved in parallel because Pternandra resembles Melastomataceae in its other wood characters.