Age and Growth in Paralarvae of the Mesopelagic Squid Abralia Trigonura Based on Daily Growth Increments in Statoliths
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1
Pacific Science (1995), vol. 49, no. 2: 143-155 © 1995 by University of Hawai'i Press. All rights reserved Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1 RICHARD EDWARD YOUNG 2 ABSTRACT: Pelagic cephalopods of the mesopelagic-boundary region in Hawai'i have proven difficult to sample but seem to occupy a variety ofhabitats within this zone. Abralia trigonura Berry inhabits the zone only as adults; A. astrosticta Berry may inhabit the inner boundary zone, and Pterygioteuthis giardi Fischer appears to be a facultative inhabitant. Three other mesopelagic species, Liocranchia reinhardti (Steenstrup), Chiroteuthis imperator Chun, and Iridoteuthis iris (Berry), are probable inhabitants; the latter two are suspected to be nonvertical migrants. The mesopelagic-boundary region also contains a variety of other pelagic cephalopods. Some are transients, common species of the mesopelagic zone in offshore waters such as Abraliopsis spp., neritic species such as Euprymna scolopes Berry, and oceanic epipelagic species such as Tremoctopus violaceus Chiaie and Argonauta argo Linnaeus. Others are appar ently permanent but either epipelagic (Onychoteuthis sp. C) or demersal (No totodarus hawaiiensis [Berry] and Haliphron atlanticus Steenstrup). Submersible observations show that Nototodarus hawaiiensis commonly "sits" on the bot tom and Haliphron atlanticus broods its young in the manner of some pelagic octopods. THE CONCEPT OF the mesopelagic-boundary over bottom depths of the same range. The region (m-b region) was first introduced by designation of an inner zone is based on Reid et al. (1991), although a general asso Reid'sfinding mesopelagic fishes resident there ciation of various mesopelagic animals with during both the day and night; mesopelagic land masses has been known for some time. -

Headed Whales (Peponocephala Electra) in Hawaiian Waters
Notes MARINE MAMMAL SCIENCE, 00(00): 00–00 (Month 2018) VC 2018 Society for Marine Mammalogy DOI: 10.1111/mms.12507 Stomach contents and diel diving behavior of melon-headed whales (Peponocephala electra) in Hawaiian waters 1 KRISTI L. WEST, Department of Human Nutrition, Food and Animal Science, College of Tropical Agriculture and Human Resources, Agricultural Sciences 216, 1955 East-West Road, University of Hawai‘i at Manoa, Honolulu, Hawai‘i 96822, U.S.A.; WILLIAM A. WALKER, Marine Mammal Laboratory, National Marine Fisheries Service, NOAA, 7600 Sand Point Way N.E., Seattle, Washington 98115, U.S.A.; ROBIN W. BAIRD,DANIEL L. WEBSTER, and GREGORY S. SCHORR, Cascadia Research Collective, 218 1=2 W. 4th Avenue, Olympia, Washington 98501, U.S.A. Knowledge of the diet and diving behavior of a species is crucial for understand- ing its behavior and ecology, and also has relevance to assessing the impact of poten- tial changes in behavior or spatial use. Assessing diet for many species of cetaceans is difficult, given that most foraging occurs far below the surface and that stomach contents of stranded animals are rarely available. Very little information on food habits or the diving behavior of melon-headed whales (Peponocephala electra)isavail- able from any region of the world. Although there is a paucity of knowledge on melon-headed whales, more is known about them in Hawaiian waters than anywhere else in the world (Aschettino et al. 2012, Woodworth et al. 2012, Baird 2016). In Hawai‘i, two populations of melon-headed whales are recognized, a Hawaiian Islands population estimated to be close to 5,000 individuals that travels offshore and among the islands, and a smaller, inshore population estimated to be about 450 individuals that is found off Hawai‘i Island and known as the Kohala resident population (Aschettino 2010, Aschettino et al. -

Eyes and Extraocular Photoreceptors in Midwater Cephalopods and Fishes: Their Roles in Detecting Downwelling Light for Counterillumination
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by OceanRep Marine Biology 51, 371-380 (1979) MARINE BIOLOGY by Springer-Verlag 1979 Eyes and Extraocular Photoreceptors in Midwater Cephalopods and Fishes: Their Roles in Detecting Downwelling Light for Counterillumination R.E. Young ], C.F.E. Roper 2 and J.F. Waiters3 ]Department of Oceanography, University of Hawaii at Manoa; Honolulu, Hawaii, USA 2Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution; Washington, D.C., USA and 3Maui Community College; Hawaii, USA Abstract The means of detecting downwelling light for counterillumination in several midwa- ter animals has been examined. Eyes and extraocular photoreceptors (dorsal photo- sensitive vesicles in the enoploteuthid squid Abraliopsis sp. B and pineal organs in the myctophid fish Mgctophum spinosum) were alternately exposed to overhead light or covered by a small opaque shield above the animal and the bioluminescent response of the animal was monitored. Covering either the eyes or the extraocular photore- ceptors resulted in a reduction in the intensity of counterillumination. Prelimi- nary experiments examining the bioluminescent feedback mechanism for monitoring intensity of bioluminescence during counterillumination in the midwater squid Abra- lia trigonura indicated that the ventral photosensitive vesicles are responsible for bioluminescent feedback. Introduction range of about 16,OO0-fold which corre- sponds to light changes that occur in Faint but highly directional daylight in clear oceanic waters over a depth range midwaters of the open ocean silhouettes of nearly 300 m (Young et al., in prepa- an opaque animal which could be visible, ration). -
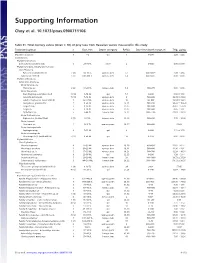
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Giant Protistan Parasites on the Gills of Cephalopods (Mollusca)
DISEASES OF AQUATIC ORGANISMS Vol. 3: 119-125. 1987 Published December 14 Dis. aquat. Org. Giant protistan parasites on the gills of cephalopods (Mollusca) Norman ~c~ean',F. G. ~ochberg~,George L. shinn3 ' Biology Department, San Diego State University, San Diego, California 92182-0057, USA Department of Invertebrate Zoology, Santa Barbara Museum of Natural History, 2559 Puesta Del Sol Road, Santa Barbara, California 93105. USA Division of Science, Northeast Missouri State University, Kirksville. Missouri 63501, USA ABSTRACT: Large Protista of unknown taxonomic affinities are described from 3 species of coleoid squids, and are reported from many other species of cephalopods. The white to yellow-orange, ovoid cyst-like parasites are partially embedded within small pockets on the surface of the gills, often in large numbers. Except for a holdfast region on one side of the large end, the surface of the parasite is elaborated into low triangular plates separated by grooves. The parasites are uninucleate; their cytoplasm bears lipid droplets and presumed paraglycogen granules. Trichocysts, present in a layer beneath the cytoplasmic surface, were found by transmission electron microscopy to be of the dino- flagellate type. Further studies are needed to clarify the taxonomic position of these protists. INTRODUCTION epoxy resin (see below). One specimen each of the coleoid squids Abralia trigonura and Histioteuthis dof- Cephalopods harbor a diversity of metazoan and leini were trawled near Oahu, Hawaii, in March, 1980. protozoan parasites (Hochberg 1983). In this study we Gill parasites from the former were fixed in formalin; used light and electron microscopy to characterize a those from the latter were fixed in osmium tetroxide. -

Factors Affecting Counterillumination As a Cryptic Strategy
Reference: Biol. Bull. 207: 1–16. (August 2004) © 2004 Marine Biological Laboratory Propagation and Perception of Bioluminescence: Factors Affecting Counterillumination as a Cryptic Strategy SO¨ NKE JOHNSEN1,*, EDITH A. WIDDER2, AND CURTIS D. MOBLEY3 1Biology Department, Duke University, Durham, North Carolina 27708; 2Marine Science Division, Harbor Branch Oceanographic Institution, Ft. Pierce, Florida 34946; and 3Sequoia Scientific Inc., Bellevue, Washington 98005 Abstract. Many deep-sea species, particularly crusta- was partially offset by the higher contrast attenuation at ceans, cephalopods, and fish, use photophores to illuminate shallow depths, which reduced the sighting distance of their ventral surfaces and thus disguise their silhouettes mismatches. This research has implications for the study of from predators viewing them from below. This strategy has spatial resolution, contrast sensitivity, and color discrimina- several potential limitations, two of which are examined tion in deep-sea visual systems. here. First, a predator with acute vision may be able to detect the individual photophores on the ventral surface. Introduction Second, a predator may be able to detect any mismatch between the spectrum of the bioluminescence and that of the Counterillumination is a common form of crypsis in the background light. The first limitation was examined by open ocean (Latz, 1995; Harper and Case, 1999; Widder, modeling the perceived images of the counterillumination 1999). Its prevalence is due to the fact that, because the of the squid Abralia veranyi and the myctophid fish Cera- downwelling light is orders of magnitude brighter than the toscopelus maderensis as a function of the distance and upwelling light, even an animal with white ventral colora- visual acuity of the viewer. -

<I>Abralia</I> (Cephalopoda)
BULLETINOF MARINESCIENCE,49(1-2): 113-136, 1991 SQUIDS OF THE GENUS ABRALIA (CEPHALOPODA) FROM THE CENTRAL EQUATORIAL PACIFIC WITH A DESCRIPTION OF ABRALIA HEMINUCHALIS, NEW SPECIES Lourdes Alvina Burgess ABSTRACT Abralia trigonura Berry, 1913, from the Hawaiian Islands is redescribed and a neotype designated. A closely related new species Abralia heminuchalis from the central equatorial Pacific is described. Material from a new area of distribution for Abralia similis Okutani and Tsuchiya, 1987, is discussed. Material for the rare Abralia astrosticta Berry, 1909, is reported, accompanied by a description of a specimen from the Marshall Islands by the late Gilbert L. Voss. All the species examined are illustrated and compared, and observations on immature animals noted. Between 1967 and 1970, while preparing a reference cephalopod collection for the Bureau of Commercial Fisheries Biological Laboratory (now the Honolulu Laboratory, Southwest Center of the National Marine Fisheries Service, National Oceanic and Atmospheric Administration, NOAA), over 5,000 oegopsid squids were identified as belonging to the genus Abralia, family Enoploteuthidae. The four species found were A. trigonura Berry, 1913, A. astrosticta Berry, 1909, and two un-named species then. One of the them has been described as A. simi/is Okutani and Tsuchiya, 1987, the other presented here as Abralia heminuchalis, n. sp. Of the more specimens identified, approximately 1,145 were A. trigonura, 2,367 A, heminuchalis, 1,484 A. similis and 96 A. astrosticta. The samples were taken mainly with a modified Cobb trawl, a 10-foot Isaacs- Kidd trawl, or a Nanaimo trawl during the cruises of several research vessels operated by the laboratory. -

<I>Abralia</I> (Cephalopoda: Enoploteuthidae)
BULLETIN OF MARINE SCIENCE, 66(2): 417–443, 2000 NEW SPECIES PAPER SQUIDS OF THE GENUS ABRALIA (CEPHALOPODA: ENOPLOTEUTHIDAE) FROM THE WESTERN TROPICAL PACIFIC WITH A DESCRIPTION OF ABRALIA OMIAE, A NEW SPECIES Kiyotaka Hidaka and Tsunemi Kubodera ABSTRACT Squids of the genus Abralia collected by a midwater trawl in the western tropical Pa- cific were examined. Five species were identified, including Abralia omiae new species. This species is characterized by having five monotypic subocular photophores, two lon- gitudinal broad bands of minute photophores on the ventral mantle, and a hectocotylized right ventral arm with two crests. The other four species identified were A. similis, A. trigonura, A. siedleckyi and A. heminuchalis. Materials from new localities for these spe- cies are discussed and comparisons to related species in several additional characters are made. Squids of the genus Abralia Gray 1849, family Enoploteuthidae, are important mem- bers in the micronektonic communities in the tropical and subtropical world oceans (e.g., Reid et al., 1991). The genus is distinguished in the family Enoploteuthidae by manus of club with one row of hooks and two rows of suckers, absence of enlarged photophores with black coverage on tips of arm IV, buccal crown with typical chromatophores on aboral surface without any other pigmentation, and five to 12 photophores on eyeball (Young et al., 1998). Several systematic studies on pelagic cephalopods in the Pacific Ocean (Quoy and Gaimard, 1832; Berry, 1909, 1913, 1914; Sasaki, 1929; Grimpe, 1931; Nesis and Nikitina, 1987; Okutani and Tsuchiya, 1987; Burgess, 1992) have revealed that nine nominal Abralia species are known from the Pacific. -

Western Central Pacific
FAOSPECIESIDENTIFICATIONGUIDEFOR FISHERYPURPOSES ISSN1020-6868 THELIVINGMARINERESOURCES OF THE WESTERNCENTRAL PACIFIC Volume2.Cephalopods,crustaceans,holothuriansandsharks FAO SPECIES IDENTIFICATION GUIDE FOR FISHERY PURPOSES THE LIVING MARINE RESOURCES OF THE WESTERN CENTRAL PACIFIC VOLUME 2 Cephalopods, crustaceans, holothurians and sharks edited by Kent E. Carpenter Department of Biological Sciences Old Dominion University Norfolk, Virginia, USA and Volker H. Niem Marine Resources Service Species Identification and Data Programme FAO Fisheries Department with the support of the South Pacific Forum Fisheries Agency (FFA) and the Norwegian Agency for International Development (NORAD) FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1998 ii The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries. M-40 ISBN 92-5-104051-6 All rights reserved. No part of this publication may be reproduced by any means without the prior written permission of the copyright owner. Applications for such permissions, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, via delle Terme di Caracalla, 00100 Rome, Italy. © FAO 1998 iii Carpenter, K.E.; Niem, V.H. (eds) FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Volume 2. Cephalopods, crustaceans, holothuri- ans and sharks. Rome, FAO. 1998. 687-1396 p. -

Cephalopoda: Teuthoidea: Enoploteuthidae
EARLY LIFE HISTORY STAGES OF ENOPLOTEUTHIN SQUIDS (CEPHALOPODA : TEUTHOIDEA : ENOPLOTEUTHIDAE) FROM HAWAIIAN WATERS R Young, R Harman To cite this version: R Young, R Harman. EARLY LIFE HISTORY STAGES OF ENOPLOTEUTHIN SQUIDS (CEPHALOPODA : TEUTHOIDEA : ENOPLOTEUTHIDAE) FROM HAWAIIAN WATERS. Vie et Milieu / Life & Environment, Observatoire Océanologique - Laboratoire Arago, 1985, pp.181-201. hal-03022097 HAL Id: hal-03022097 https://hal.sorbonne-universite.fr/hal-03022097 Submitted on 24 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. VIE MILIEU, 1985, 35 (3/4) : 181-201 EARLY LIFE HISTORY STAGES OF ENOPLOTEUTHIN SQUIDS (CEPHALOPODA : TEUTHOIDEA : ENOPLOTEUTHIDAE) FROM HAWAIIAN WATERS R.E. YOUNG and R.F. HARMAN Department of Oceanography, University of Hawaii, 1000 Pope Road, Honolulu, Hawaii 96822 USA HAWAII ABSTRACT. — Species of the enoploteuthid subfamily Enoploteuthinae spawn LARVAE individual eggs in the plankton. Eggs captured off Hawaii were reared in the CEPHALOPODA laboratory for several days after hatching. The hatchings were matched to size-series ENOPLOTEUTHIDAE of larvae taken from an extensive trawling program designed to catch squid larvae. SQUIDS The early life history stages of thèse species are described and systematic characters VERTICAL-DISTRIBUTION evaluated. -

Table of Contents
SPECIAL ISSUE NUMBER-2 (2017) Print ISSN: 0974-6455 Online ISSN: 2321-4007 CODEN: BBRCBA www.bbrc.in University Grants Commission (UGC) New Delhi, India Approved Journal An International Peer Reviewed Open Access Journal For Rapid Publication Registered with the Registrar of Newspapers for India under Reg. No. 498/2007 Bioscience Biotechnology Research Communications SPECIAL ISSUE NUMBER-2 (2017) Design, implementation and data analysis of a comprehensive screening scheme of common non-communicable diseases in some semi-public organizations in Tehran in 2016 Behshad Alimadadi Jani and Ahmad Shafaeizadeh 1-6 Esophageal cancer: the changes in incidence during last years Mohammad Yaqub Rajput 7-11 The relationship between metacognitive and self-efficacy beliefs with test anxiety and academic achievement of students Adis Kraskian Mujembari (PhD) and Fatemeh Jahedtabar (MA) 12-21 Examination of underground tunnels for vulnerability against water entrance into the tunnel and proper approaches to cope them Mina Aligholi 22-28 Importance of providing electrical harmony performance standards during use of hospital medical equipments Hossein Shokrekhoda 29-32 Illness perception and self-care behavior in patients with myocardial infarction Leila Ahmadi Ghahnaviyeh, Reza Bagherian, Awat Feizi, Atefeh Afshari and Firoozeh Mostafavi Darani 33-38 Evaluation of correlation between lower jaw and lip rate of paresthesia and inferior alveolar canal diameter changes after mandibular fracture Anis Moradi and Seyed Mehdi Hosseini 39-45 Prevalence of tachydysrhythmia -

Amendment 4 to the Fishery Ecosystem Plan for the Hawaii Archipelago
Amendment 4 to the Fishery Ecosystem Plan for the Hawaii Archipelago Revised Descriptions and Identification of Essential Fish Habitat and Habitat Areas of Particular Concern for Bottomfish and Seamount Groundfish of the Hawaiian Archipelago January 28, 2016 1 Responsible Agencies The Western Pacific Regional Fishery Management Council was established by the Magnuson- Stevens Fishery and Conservation Management Act (MSA) to develop management plans for U.S. fisheries operating in the offshore waters around the territories of American Samoa and Guam, the State of Hawai‘i, the Commonwealth of the Northern Mariana Islands, and the U.S. Pacific Remote Island Areas (PRIA; Palmyra Atoll, Kingman Reef, Jarvis Island, Baker Island, Howland Island, Johnston Atoll, and Wake Island). The territories, commonwealth, state, and PRIA are collectively the western Pacific Region. Once a plan is approved by the Secretary of Commerce, it is implemented as appropriate by federal regulations that are enforced by NMFS and the U.S. Coast Guard, in cooperation with state, territorial, and commonwealth agencies. For further information contact: Kitty M. Simonds Michael D. Tosatto Executive Director Regional Administrator Western Pacific Regional Fishery Pacific Islands Regional Office Management Council National Marine Fisheries Service 1164 Bishop St., Suite 1400 1845 Wasp Blvd., Bldg 176 Honolulu, HI 96813 Honolulu, HI 96818 (808) 522-8220 (808) 725-5000 List of Preparers This document was prepared by (in alphabetical order): Matt Dunlap, NMFS Pacific Islands