Headed Whales (Peponocephala Electra) in Hawaiian Waters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

Woodsia Obtusa
COSEWIC Assessment and Update Status Report on the blunt-lobed woodsia Woodsia obtusa in Canada THREATENED 2007 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2006. COSEWIC assessment and update status report on the blunt-lobed woodsia Woodsia obtusa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 17 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report: COSEWIC 2000. COSEWIC assessment and status report on the blunt-lobed woodsia Woodsia obtusa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-20 pp. Consaul, L.L. 1994. COSEWIC status report on the blunt-lobed woodsia Woodsia obtusa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-20 pp. Production note: COSEWIC would like to acknowledge Matthew Wild for writing the status report on the blunt-lobed woodsia Woodsia obtusa in Canada, prepared under contract with Environment Canada, overseen and edited by Dr. Erich Haber, Co-chair, COSEWIC Plants and Lichens Species Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la woodsie à lobes arrondis (Woodsia obtusa) au Canada – Mise à jour. -

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1
Pacific Science (1995), vol. 49, no. 2: 143-155 © 1995 by University of Hawai'i Press. All rights reserved Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1 RICHARD EDWARD YOUNG 2 ABSTRACT: Pelagic cephalopods of the mesopelagic-boundary region in Hawai'i have proven difficult to sample but seem to occupy a variety ofhabitats within this zone. Abralia trigonura Berry inhabits the zone only as adults; A. astrosticta Berry may inhabit the inner boundary zone, and Pterygioteuthis giardi Fischer appears to be a facultative inhabitant. Three other mesopelagic species, Liocranchia reinhardti (Steenstrup), Chiroteuthis imperator Chun, and Iridoteuthis iris (Berry), are probable inhabitants; the latter two are suspected to be nonvertical migrants. The mesopelagic-boundary region also contains a variety of other pelagic cephalopods. Some are transients, common species of the mesopelagic zone in offshore waters such as Abraliopsis spp., neritic species such as Euprymna scolopes Berry, and oceanic epipelagic species such as Tremoctopus violaceus Chiaie and Argonauta argo Linnaeus. Others are appar ently permanent but either epipelagic (Onychoteuthis sp. C) or demersal (No totodarus hawaiiensis [Berry] and Haliphron atlanticus Steenstrup). Submersible observations show that Nototodarus hawaiiensis commonly "sits" on the bot tom and Haliphron atlanticus broods its young in the manner of some pelagic octopods. THE CONCEPT OF the mesopelagic-boundary over bottom depths of the same range. The region (m-b region) was first introduced by designation of an inner zone is based on Reid et al. (1991), although a general asso Reid'sfinding mesopelagic fishes resident there ciation of various mesopelagic animals with during both the day and night; mesopelagic land masses has been known for some time. -

An Illustrated Key to the Families of the Order
CLYDE F. E. ROP An Illustrated RICHARD E. YOl and GILBERT L. VC Key to the Families of the Order Teuthoidea Cephalopoda) SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • 1969 NUMBER 13 SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY NUMBER 13 Clyde F. E. Roper, An Illustrated Key 5K?Z" to the Families of the Order Teuthoidea (Cephalopoda) SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON 1969 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Institution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge not strictly professional." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. -

Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries
Advances in Cephalopod Science:Biology, Ecology, Cultivation and Fisheries,Vol 67 (2014) Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book Advances in Marine Biology, Vol. 67 published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: Paul G.K. Rodhouse, Graham J. Pierce, Owen C. Nichols, Warwick H.H. Sauer, Alexander I. Arkhipkin, Vladimir V. Laptikhovsky, Marek R. Lipiński, Jorge E. Ramos, Michaël Gras, Hideaki Kidokoro, Kazuhiro Sadayasu, João Pereira, Evgenia Lefkaditou, Cristina Pita, Maria Gasalla, Manuel Haimovici, Mitsuo Sakai and Nicola Downey. Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries. In Erica A.G. Vidal, editor: Advances in Marine Biology, Vol. 67, Oxford: United Kingdom, 2014, pp. 99-233. ISBN: 978-0-12-800287-2 © Copyright 2014 Elsevier Ltd. Academic Press Advances in CephalopodAuthor's Science:Biology, personal Ecology, copy Cultivation and Fisheries,Vol 67 (2014) CHAPTER TWO Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries Paul G.K. -

Vertical Distribution Patterns of Cephalopods in the Northern Gulf of Mexico
fmars-07-00047 February 20, 2020 Time: 15:34 # 1 ORIGINAL RESEARCH published: 21 February 2020 doi: 10.3389/fmars.2020.00047 Vertical Distribution Patterns of Cephalopods in the Northern Gulf of Mexico Heather Judkins1* and Michael Vecchione2 1 Department of Biological Sciences, University of South Florida St. Petersburg, St. Petersburg, FL, United States, 2 NMFS National Systematics Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, DC, United States Cephalopods are important in midwater ecosystems of the Gulf of Mexico (GOM) as both predator and prey. Vertical distribution and migration patterns (both diel and ontogenic) are not known for the majority of deep-water cephalopods. These varying patterns are of interest as they have the potential to contribute to the movement of large amounts of nutrients and contaminants through the water column during diel migrations. This can be of particular importance if the migration traverses a discrete layer with particular properties, as happened with the deep-water oil plume located between 1000 and 1400 m during the Deepwater Horizon (DWH) oil spill. Two recent studies focusing on the deep-water column of the GOM [2011 Offshore Nekton Sampling and Edited by: Jose Angel Alvarez Perez, Analysis Program (ONSAP) and 2015–2018 Deep Pelagic Nekton Dynamics of the Gulf Universidade do Vale do Itajaí, Brazil of Mexico (DEEPEND)] program, produced a combined dataset of over 12,500 midwater Reviewed by: cephalopod records for the northern GOM region. We summarize vertical distribution Helena Passeri Lavrado, 2 Federal University of Rio de Janeiro, patterns of cephalopods from the cruises that utilized a 10 m Multiple Opening/Closing Brazil Net and Environmental Sensing System (MOC10). -

Ommastrephidae 199
click for previous page Decapodiformes: Ommastrephidae 199 OMMASTREPHIDAE Flying squids iagnostic characters: Medium- to Dlarge-sized squids. Funnel locking appara- tus with a T-shaped groove. Paralarvae with fused tentacles. Arms with biserial suckers. Four rows of suckers on tentacular clubs (club dactylus with 8 sucker series in Illex). Hooks never present hooks never on arms or clubs. One of the ventral pair of arms present usually hectocotylized in males. Buccal connec- tives attach to dorsal borders of ventral arms. Gladius distinctive, slender. funnel locking apparatus with Habitat, biology, and fisheries: Oceanic and T-shaped groove neritic. This is one of the most widely distributed and conspicuous families of squids in the world. Most species are exploited commercially. Todarodes pacificus makes up the bulk of the squid landings in Japan (up to 600 000 t annually) and may comprise at least 1/2 the annual world catch of cephalopods.In various parts of the West- ern Central Atlantic, 6 species of ommastrephids currently are fished commercially or for bait, or have a potential for exploitation. Ommastrephids are powerful swimmers and some species form large schools. Some neritic species exhibit strong seasonal migrations, wherein they occur in huge numbers in inshore waters where they are accessable to fisheries activities. The large size of most species (commonly 30 to 50 cm total length and up to 120 cm total length) and the heavily mus- cled structure, make them ideal for human con- ventral view sumption. Similar families occurring in the area Onychoteuthidae: tentacular clubs with claw-like hooks; funnel locking apparatus a simple, straight groove. -

Cephalopoda of the North Atlantic: the Family
RICHARD E. TOUNG A Monograph of the LYDE F. E. ROPER ^ r 7 l r ' 1 Cephalopoda of the North Atlantic: The Family SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • 1969 NUMBER 5 SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY NUMBER 5 Richard E. Young A Monograph of the and Clyde F. E. Roper ^ 1 1 -• r •» Cephalopoda ot the North Atlantic: The Family Cycloteuthidae SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Insti- tution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge not strictly professional." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smith- sonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. -
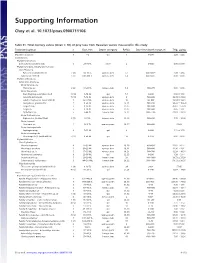
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Giant Protistan Parasites on the Gills of Cephalopods (Mollusca)
DISEASES OF AQUATIC ORGANISMS Vol. 3: 119-125. 1987 Published December 14 Dis. aquat. Org. Giant protistan parasites on the gills of cephalopods (Mollusca) Norman ~c~ean',F. G. ~ochberg~,George L. shinn3 ' Biology Department, San Diego State University, San Diego, California 92182-0057, USA Department of Invertebrate Zoology, Santa Barbara Museum of Natural History, 2559 Puesta Del Sol Road, Santa Barbara, California 93105. USA Division of Science, Northeast Missouri State University, Kirksville. Missouri 63501, USA ABSTRACT: Large Protista of unknown taxonomic affinities are described from 3 species of coleoid squids, and are reported from many other species of cephalopods. The white to yellow-orange, ovoid cyst-like parasites are partially embedded within small pockets on the surface of the gills, often in large numbers. Except for a holdfast region on one side of the large end, the surface of the parasite is elaborated into low triangular plates separated by grooves. The parasites are uninucleate; their cytoplasm bears lipid droplets and presumed paraglycogen granules. Trichocysts, present in a layer beneath the cytoplasmic surface, were found by transmission electron microscopy to be of the dino- flagellate type. Further studies are needed to clarify the taxonomic position of these protists. INTRODUCTION epoxy resin (see below). One specimen each of the coleoid squids Abralia trigonura and Histioteuthis dof- Cephalopods harbor a diversity of metazoan and leini were trawled near Oahu, Hawaii, in March, 1980. protozoan parasites (Hochberg 1983). In this study we Gill parasites from the former were fixed in formalin; used light and electron microscopy to characterize a those from the latter were fixed in osmium tetroxide. -

Factors Affecting Counterillumination As a Cryptic Strategy
Reference: Biol. Bull. 207: 1–16. (August 2004) © 2004 Marine Biological Laboratory Propagation and Perception of Bioluminescence: Factors Affecting Counterillumination as a Cryptic Strategy SO¨ NKE JOHNSEN1,*, EDITH A. WIDDER2, AND CURTIS D. MOBLEY3 1Biology Department, Duke University, Durham, North Carolina 27708; 2Marine Science Division, Harbor Branch Oceanographic Institution, Ft. Pierce, Florida 34946; and 3Sequoia Scientific Inc., Bellevue, Washington 98005 Abstract. Many deep-sea species, particularly crusta- was partially offset by the higher contrast attenuation at ceans, cephalopods, and fish, use photophores to illuminate shallow depths, which reduced the sighting distance of their ventral surfaces and thus disguise their silhouettes mismatches. This research has implications for the study of from predators viewing them from below. This strategy has spatial resolution, contrast sensitivity, and color discrimina- several potential limitations, two of which are examined tion in deep-sea visual systems. here. First, a predator with acute vision may be able to detect the individual photophores on the ventral surface. Introduction Second, a predator may be able to detect any mismatch between the spectrum of the bioluminescence and that of the Counterillumination is a common form of crypsis in the background light. The first limitation was examined by open ocean (Latz, 1995; Harper and Case, 1999; Widder, modeling the perceived images of the counterillumination 1999). Its prevalence is due to the fact that, because the of the squid Abralia veranyi and the myctophid fish Cera- downwelling light is orders of magnitude brighter than the toscopelus maderensis as a function of the distance and upwelling light, even an animal with white ventral colora- visual acuity of the viewer.