Cephalopoda: Teuthoidea: Enoploteuthidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crappie and Crappie Fishing
Crappie & Crappie Fishing Crappie are among the most popular sport fishes in Texas. They are known by various names including white perch, sac-a-lait, calico bass, and paper-mouth. Two species are found in Texas, the white crappie (Pomoxis annularis) and black crappie (P. nigromaculatus). Black crap pie have irregular dark speck les and blotches on their sides. On white crappie, the dark markings consist of regularly arranged vertical bars. When in doubt, count the number of sharp dorsal spines at the front of a crappie’s dorsal fin. Black crappie have seven or eight spines while white crappie Young crappie feed on microscopic crustaceans called have five or six. During the spawning season, males of zooplankton. Juveniles and adults feed primarily on both species develop dark markings over most of the small threadfin and gizzard shad and insect larvae, es body, causing many anglers to misidentify male white pecially mayflies. Their diet also includes minnows, crappie as black crappie. silversides, other crappie and any other fish small enough to swallow. Black crappie are more numerous in the clear, acidic to slightly alkaline waters of East Texas. White crappie are found state In lakes with low bass populations, crappie often wide. Fish of both species may live up to eight years and overpopulate and become stunted. For crappie to reach become sexually mature at one to two years. Crappie belong larger sizes, populations must experience high total mor to the same family as the sunfishes and black basses; like tality to keep their numbers within the carrying capacity their cousins, crappie are nest builders. -

Fish Spawning Aggregations
Fish Spawning Aggregations a focal point of fisheries management and marine conservation in Mexico Photo: Octavio Aburto Authorship Brad Erisman – Coastal Fisheries Research Program, University of Texas Marine Science Institute, 750 Channel View Drive, Port Aransas, TX 78373 William Heyman – LGL Ecological Research Associates, Inc., 4103 S. Texas Avenue, Bryan TX 77802 Stuart Fulton – Comunidad y Biodiversidad, Isla del Peruano 215, Lomas de Miramar, Guaymas, Sonora, Mexico Timothy Rowell – Gulf of California Marine Program, Scripps Institution of Oceanography, 9500 Gilman Drive, La Jolla, CA 92037 Illustrations – Larry Allen and Madeline Wukusick Graphic Design – Madeline Wukusick | www.communique.design Photography – Octavio Aburto, Richard Barnden, Douglas David Seifert, Walt Stearns, Cristina Limonta, Alfredo Barroso Citation – Erisman, B., W.D. Heyman, S. Fulton, and T.Rowell 2018. Fish spawning aggregations: a focal point of fisheries management and marine conservation in Mexico. Gulf of California Marine Program, La Jolla, CA. 24 p. Email Contact: Brad Erisman, [email protected] Fish Spawning Aggregations // 2 Contents > Introduction .................................................................................................................................................................. 4 > What are fish spawning aggregations (FSAs)? ............................................................................................................ 5 > What kinds of fishes form FSAs? ................................................................................................................................ -

Largemouth Bass Biology and Life History
SRAC Publication No. 200 August 1997 VI PR Revision Largemouth Bass Biology and Life History James T. Davis and Joe T. Lock* The largemouth bass (Micropterus Largemouth bass will eat a variety salmoides) is one of several “bass- of live fish, but bluegill are partic- es” that are actually members of ularly important in ponds and the sunfish family. There are two small lakes because they repro- recognized subspecies, the duce throughout the warm Florida and the Northern, which months. This furnishes a continual will blend genetically. Although supply of different size forage. the two subspecies differ slightly Tilapia* and/or goldfish are com- in body structure, behavior, and monly used as forage on fish growth, biochemical tests are nec- farms and in intensively managed essary to positively identify them. Largemouth bass. lakes because more can be pro- duced at lower cost. About 5 Food and growth reflex action toward anything that pounds of live forage are required moves. (The bass motto: If food is for annual maintenance, and 10 Largemouth bass are valued by there, eat it.) pounds of forage are required to fishermen chiefly because of their add 1 pound of gain to large- The availability of adequate size fighting ability. They are vora- mouth bass. cious predators that readily strike live food (baitfish or forage) usu- artificial baits. Bass begin to eat ally limits bass growth. With ade- The swimming speed of large- fish when they are about 2 inches quate forage, largemouth bass can mouth bass has not been studied long. They swallow live fish and surpass 2 pounds the first year, in depth. -

Coral Reef Fish Spawning Periodicity and Habitat in New Caledonia: a Multi-Faceted Approach in a Data-Deficient Environment
Coral Reef Fish Spawning Periodicity and Habitat in New Caledonia: a multi-faceted approach in a data-deficient environment Adrian FLYNN1*, Sébastien SARRAMEGNA2 and Michel KULBICKI3 1Hydrobiology Pty Ltd. 47 Park Rd. PO Box 2050 Milton 4064 Queensland, Australia 2 Falconbridge NC SAS, 9, rue d'Austerlitz BP MGA08 98802 Nouméa Cedex, Nouvelle-Calédonie 3 Institut de recherche pour le développment, BP A5 98848 Nouméa Cedex, Nouvelle-Calédonie *Corresponding Author: A. Flynn e-mail: [email protected] Abstract An Environmental Impact Assessment Introduction (EIA) for a proposed mining project on the west coast While most temperate fishes have a well-defined of Northern Province, New Caledonia, required an breeding season that is regulated by hormonal changes understanding of coral reef fish spawning/aggregation and a variety of environmental cues such as periodicity and habitat utilisation in New Caledonia in temperature and photoperiod (Scott 1979; Lam 1983; order to describe and mitigate the potential impacts of Bye 1984; Stacey 1984), tropical species generally the development. A study was undertaken that have a protracted breeding season and the specific encompassed literature review, interpretation of cues regulating spawning periodicity are not well oceanographic data, analysis of gonad index data known, although photoperiod, sea temperature and spanning some 18 years, analysis of commercial currents are often quoted as the most influential fisheries production data, interpretation of sales data (Munro et al. 1973; Thresher 1984; Walsh 1987). from the Nouméa fish market, interviews with Although the timing of spawning can occur commercial and subsistence fishermen and personal randomly in tropical environments, spawning is more communication with researchers at University of New commonly synchronised within a population Caledonia regarding unpublished records and (Johannes 1978; Colin and Clavijo 1988). -

The Cephalopoda
Carl Chun THE CEPHALOPO PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Carl Chun THE CEPHALOPODA NOTE TO PLATE LXVIII Figure 7 should read Figure 8 Figure 9 should read Figure 7 GERMAN DEEPSEA EXPEDITION 1898-1899. VOL. XVIII SCIENTIFIC RESULTS QF THE GERMAN DEEPSEA EXPEDITION ON BOARD THE*STEAMSHIP "VALDIVIA" 1898-1899 Volume Eighteen UNDER THE AUSPICES OF THE GERMAN MINISTRY OF THE INTERIOR Supervised by CARL CHUN, Director of the Expedition Professor of Zoology , Leipzig. After 1914 continued by AUGUST BRAUER Professor of Zoology, Berlin Carl Chun THE CEPHALOPODA PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Translatedfrom the German ISRAEL PROGRAM FOR SCIENTIFIC TRANSLATIONS Jerusalem 1975 TT 69-55057/2 Published Pursuant to an Agreement with THE SMITHSONIAN INSTITUTION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D.C. Since the study of the Cephalopoda is a very specialized field with a unique and specific terminology and phrase- ology, it was necessary to edit the translation in a technical sense to insure that as accurate and meaningful a represen- tation of Chun's original work as possible would be achieved. We hope to have accomplished this responsibility. Clyde F. E. Roper and Ingrid H. Roper Technical Editors Copyright © 1975 Keter Publishing House Jerusalem Ltd. IPST Cat. No. 05452 8 ISBN 7065 1260 X Translated by Albert Mercado Edited by Prof. O. Theodor Copy-edited by Ora Ashdit Composed, Printed and Bound by Keterpress Enterprises, Jerusalem, Israel Available from the U. S. DEPARTMENT OF COMMERCE National Technical Information Service Springfield, Va. 22151 List of Plates I Thaumatolampas diadema of luminous o.rgans 95 luminous organ 145 n.gen.n.sp. -

Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1
Pacific Science (1995), vol. 49, no. 2: 143-155 © 1995 by University of Hawai'i Press. All rights reserved Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1 RICHARD EDWARD YOUNG 2 ABSTRACT: Pelagic cephalopods of the mesopelagic-boundary region in Hawai'i have proven difficult to sample but seem to occupy a variety ofhabitats within this zone. Abralia trigonura Berry inhabits the zone only as adults; A. astrosticta Berry may inhabit the inner boundary zone, and Pterygioteuthis giardi Fischer appears to be a facultative inhabitant. Three other mesopelagic species, Liocranchia reinhardti (Steenstrup), Chiroteuthis imperator Chun, and Iridoteuthis iris (Berry), are probable inhabitants; the latter two are suspected to be nonvertical migrants. The mesopelagic-boundary region also contains a variety of other pelagic cephalopods. Some are transients, common species of the mesopelagic zone in offshore waters such as Abraliopsis spp., neritic species such as Euprymna scolopes Berry, and oceanic epipelagic species such as Tremoctopus violaceus Chiaie and Argonauta argo Linnaeus. Others are appar ently permanent but either epipelagic (Onychoteuthis sp. C) or demersal (No totodarus hawaiiensis [Berry] and Haliphron atlanticus Steenstrup). Submersible observations show that Nototodarus hawaiiensis commonly "sits" on the bot tom and Haliphron atlanticus broods its young in the manner of some pelagic octopods. THE CONCEPT OF the mesopelagic-boundary over bottom depths of the same range. The region (m-b region) was first introduced by designation of an inner zone is based on Reid et al. (1991), although a general asso Reid'sfinding mesopelagic fishes resident there ciation of various mesopelagic animals with during both the day and night; mesopelagic land masses has been known for some time. -

Biodiversity of Cephalopod Early-Life Stages Across the Southeastern Brazilian Bight: Spatio-Temporal Patterns in Taxonomic Richness
Marine Biodiversity https://doi.org/10.1007/s12526-019-00980-w ORIGINAL PAPER Biodiversity of cephalopod early-life stages across the Southeastern Brazilian Bight: spatio-temporal patterns in taxonomic richness Carolina C. Araújo1,2 & Maria A. Gasalla1,2 Received: 15 October 2018 /Revised: 20 May 2019 /Accepted: 5 June 2019 # Senckenberg Gesellschaft für Naturforschung 2019 Abstract The diversity patterns of cephalopod early-life stages on the continental shelf of Southeastern Brazilian Bight (SBB, 22–25°S) were investigated using a historical plankton archive of 22 oceanographic cruises carried out from 1974 to 2010. From 874 plankton samples, 438 were positive for cephalopod paralarvae (n = 2116), which were identified to the lowest taxonomic level possible, totaling 15 taxa belonging to 11 families. Richness and diversity indexes (Shannon-Wiener, Simpson, Pielou’seven- ness) revealed a cross-shelf gradient, independent of season and latitude. Abundance k-dominance curves were consistent with this depth-related trend, resulting in high values of k-dominance for the inner shelf during both summer and winter. Two major assemblages were identified by cluster analyses: an inner shelf and a mid-outer shelf. During summer, the inner shelf assemblage was composed of neritic Loliginidae Lesueur, 1821 and epipelagic Argonautidae Tryon, 1879, while in winter, benthic Octopodidae Orbigny, 1840 replaced Argonautidae in importance. These data reveal a remarkable difference in Argonautidae and Octopodidae paralarvae abundance, suggesting a seasonal reproductive pattern for these cephalopods in the SBB. Mesopelagic Enoploteuthidae Pfeffer, 1900 and Ommastrephidae Steenstrup, 1857 characterized the mid-outer shelf assem- blages both in summer and winter. Although based on a higher taxonomic level, the distribution of cephalopod paralarva families reflected not only oceanographic patterns of the SBB but also their adaptations and reproductive strategies. -

An Illustrated Key to the Families of the Order
CLYDE F. E. ROP An Illustrated RICHARD E. YOl and GILBERT L. VC Key to the Families of the Order Teuthoidea Cephalopoda) SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • 1969 NUMBER 13 SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY NUMBER 13 Clyde F. E. Roper, An Illustrated Key 5K?Z" to the Families of the Order Teuthoidea (Cephalopoda) SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON 1969 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Institution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge not strictly professional." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. -

Headed Whales (Peponocephala Electra) in Hawaiian Waters
Notes MARINE MAMMAL SCIENCE, 00(00): 00–00 (Month 2018) VC 2018 Society for Marine Mammalogy DOI: 10.1111/mms.12507 Stomach contents and diel diving behavior of melon-headed whales (Peponocephala electra) in Hawaiian waters 1 KRISTI L. WEST, Department of Human Nutrition, Food and Animal Science, College of Tropical Agriculture and Human Resources, Agricultural Sciences 216, 1955 East-West Road, University of Hawai‘i at Manoa, Honolulu, Hawai‘i 96822, U.S.A.; WILLIAM A. WALKER, Marine Mammal Laboratory, National Marine Fisheries Service, NOAA, 7600 Sand Point Way N.E., Seattle, Washington 98115, U.S.A.; ROBIN W. BAIRD,DANIEL L. WEBSTER, and GREGORY S. SCHORR, Cascadia Research Collective, 218 1=2 W. 4th Avenue, Olympia, Washington 98501, U.S.A. Knowledge of the diet and diving behavior of a species is crucial for understand- ing its behavior and ecology, and also has relevance to assessing the impact of poten- tial changes in behavior or spatial use. Assessing diet for many species of cetaceans is difficult, given that most foraging occurs far below the surface and that stomach contents of stranded animals are rarely available. Very little information on food habits or the diving behavior of melon-headed whales (Peponocephala electra)isavail- able from any region of the world. Although there is a paucity of knowledge on melon-headed whales, more is known about them in Hawaiian waters than anywhere else in the world (Aschettino et al. 2012, Woodworth et al. 2012, Baird 2016). In Hawai‘i, two populations of melon-headed whales are recognized, a Hawaiian Islands population estimated to be close to 5,000 individuals that travels offshore and among the islands, and a smaller, inshore population estimated to be about 450 individuals that is found off Hawai‘i Island and known as the Kohala resident population (Aschettino 2010, Aschettino et al. -

Eyes and Extraocular Photoreceptors in Midwater Cephalopods and Fishes: Their Roles in Detecting Downwelling Light for Counterillumination
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by OceanRep Marine Biology 51, 371-380 (1979) MARINE BIOLOGY by Springer-Verlag 1979 Eyes and Extraocular Photoreceptors in Midwater Cephalopods and Fishes: Their Roles in Detecting Downwelling Light for Counterillumination R.E. Young ], C.F.E. Roper 2 and J.F. Waiters3 ]Department of Oceanography, University of Hawaii at Manoa; Honolulu, Hawaii, USA 2Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution; Washington, D.C., USA and 3Maui Community College; Hawaii, USA Abstract The means of detecting downwelling light for counterillumination in several midwa- ter animals has been examined. Eyes and extraocular photoreceptors (dorsal photo- sensitive vesicles in the enoploteuthid squid Abraliopsis sp. B and pineal organs in the myctophid fish Mgctophum spinosum) were alternately exposed to overhead light or covered by a small opaque shield above the animal and the bioluminescent response of the animal was monitored. Covering either the eyes or the extraocular photore- ceptors resulted in a reduction in the intensity of counterillumination. Prelimi- nary experiments examining the bioluminescent feedback mechanism for monitoring intensity of bioluminescence during counterillumination in the midwater squid Abra- lia trigonura indicated that the ventral photosensitive vesicles are responsible for bioluminescent feedback. Introduction range of about 16,OO0-fold which corre- sponds to light changes that occur in Faint but highly directional daylight in clear oceanic waters over a depth range midwaters of the open ocean silhouettes of nearly 300 m (Young et al., in prepa- an opaque animal which could be visible, ration). -
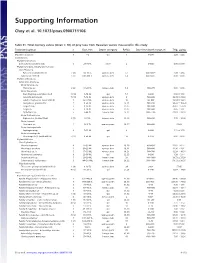
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Giant Protistan Parasites on the Gills of Cephalopods (Mollusca)
DISEASES OF AQUATIC ORGANISMS Vol. 3: 119-125. 1987 Published December 14 Dis. aquat. Org. Giant protistan parasites on the gills of cephalopods (Mollusca) Norman ~c~ean',F. G. ~ochberg~,George L. shinn3 ' Biology Department, San Diego State University, San Diego, California 92182-0057, USA Department of Invertebrate Zoology, Santa Barbara Museum of Natural History, 2559 Puesta Del Sol Road, Santa Barbara, California 93105. USA Division of Science, Northeast Missouri State University, Kirksville. Missouri 63501, USA ABSTRACT: Large Protista of unknown taxonomic affinities are described from 3 species of coleoid squids, and are reported from many other species of cephalopods. The white to yellow-orange, ovoid cyst-like parasites are partially embedded within small pockets on the surface of the gills, often in large numbers. Except for a holdfast region on one side of the large end, the surface of the parasite is elaborated into low triangular plates separated by grooves. The parasites are uninucleate; their cytoplasm bears lipid droplets and presumed paraglycogen granules. Trichocysts, present in a layer beneath the cytoplasmic surface, were found by transmission electron microscopy to be of the dino- flagellate type. Further studies are needed to clarify the taxonomic position of these protists. INTRODUCTION epoxy resin (see below). One specimen each of the coleoid squids Abralia trigonura and Histioteuthis dof- Cephalopods harbor a diversity of metazoan and leini were trawled near Oahu, Hawaii, in March, 1980. protozoan parasites (Hochberg 1983). In this study we Gill parasites from the former were fixed in formalin; used light and electron microscopy to characterize a those from the latter were fixed in osmium tetroxide.