Biodiversity of Cephalopod Early-Life Stages Across the Southeastern Brazilian Bight: Spatio-Temporal Patterns in Taxonomic Richness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cephalopoda
Carl Chun THE CEPHALOPO PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Carl Chun THE CEPHALOPODA NOTE TO PLATE LXVIII Figure 7 should read Figure 8 Figure 9 should read Figure 7 GERMAN DEEPSEA EXPEDITION 1898-1899. VOL. XVIII SCIENTIFIC RESULTS QF THE GERMAN DEEPSEA EXPEDITION ON BOARD THE*STEAMSHIP "VALDIVIA" 1898-1899 Volume Eighteen UNDER THE AUSPICES OF THE GERMAN MINISTRY OF THE INTERIOR Supervised by CARL CHUN, Director of the Expedition Professor of Zoology , Leipzig. After 1914 continued by AUGUST BRAUER Professor of Zoology, Berlin Carl Chun THE CEPHALOPODA PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Translatedfrom the German ISRAEL PROGRAM FOR SCIENTIFIC TRANSLATIONS Jerusalem 1975 TT 69-55057/2 Published Pursuant to an Agreement with THE SMITHSONIAN INSTITUTION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D.C. Since the study of the Cephalopoda is a very specialized field with a unique and specific terminology and phrase- ology, it was necessary to edit the translation in a technical sense to insure that as accurate and meaningful a represen- tation of Chun's original work as possible would be achieved. We hope to have accomplished this responsibility. Clyde F. E. Roper and Ingrid H. Roper Technical Editors Copyright © 1975 Keter Publishing House Jerusalem Ltd. IPST Cat. No. 05452 8 ISBN 7065 1260 X Translated by Albert Mercado Edited by Prof. O. Theodor Copy-edited by Ora Ashdit Composed, Printed and Bound by Keterpress Enterprises, Jerusalem, Israel Available from the U. S. DEPARTMENT OF COMMERCE National Technical Information Service Springfield, Va. 22151 List of Plates I Thaumatolampas diadema of luminous o.rgans 95 luminous organ 145 n.gen.n.sp. -

Abra/Iopsis Pacificus, a New Species of the Squid Family Enoploteuthidae from the Northwest Pacific
Bull. Natn. Sci. Mus., Tokyo, Ser. A, 16(2), pp. 47-60, June 22, 1990 Abra/iopsis pacificus, a New Species of the Squid Family Enoploteuthidae from the Northwest Pacific By Kotaro TSUCHIYA and Takashi OKUTANI Tokyo University of Fisheries, Tokyo Abstract Abraliopsis (Abraliopsis) pacificus n. sp. is described. This species is char acterized by having diffused photophore arrangement on head, developed carpal flap and aboral keel on tentacular club, unequal offsetcrests on hectocotylus and rectangular arm sucker ring teeth. It is distributed commonly in the mid water of Northwest Pacific Basin. During the systematic study on the Family Enoploteuthidae mainly based on the midwater samples of the R/V Kaiyo-Maru from the Northwest Pacific Ocean, some new facts including undescribed species have been discovered. The previous paper described a new species of the genus Abralia GRAY, 1849, A. similis OKUTANI et Tsu CHIYA, 1987, and here another new species of the genus Abraliopsis JouBIN, 1896 is described. This series of study is aiming at not only describing new taxa of this diverse family, but also making a critical revision of the family Enoploteuthidae. The nominal species of the genus Abraliopsis hitherto known are as follows: Abraliopsis hoylei (PFEFFER, 1884): Western Indian Ocean Abraliopsis lineata (GOODRICH, 1896): Indian Ocean Abraliopsis pfefferi JoUBIN, 1896: Atlantic Abraliopsis ajfinis (PFEFFER, 1912): Eastern Tropical Pacific Abrailopsis gilchristi (ROBSON, 1924): South Africa and New Zealand Abraliopsis felis McGOWAN et OKUTANI, 1968: Northern North Pacific, Transitional Abraliopsis falco YOUNG, 1972: California Current to Northwest Pacific Abraliopsis tui RIDDELL, 1985: New Zealand Abraliopsis chuni NEsrs, 1982: Western Indian Ocean Abraliopsis atlantica NESIS, 1982: Tropical and subtropical Atlantic In addition to this, OKUTANI (1985) has early recognized the existence of an undescribed species of this genus in the Northwest Pacific. -

Systematics and Distribution of Abraliopsis (Cephalopoda : Enoploteuthidae) in Australian Waters
University of Wollongong Research Online Faculty of Science, Medicine & Health - Honours Theses University of Wollongong Thesis Collections 2015 Systematics and Distribution of Abraliopsis (Cephalopoda : Enoploteuthidae) in Australian Waters Kwan Wah Li Follow this and additional works at: https://ro.uow.edu.au/thsci University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Li, Kwan Wah, Systematics and Distribution of Abraliopsis (Cephalopoda : Enoploteuthidae) in Australian Waters, BEnviSci Hons, School of Earth & Environmental Sciences, University of Wollongong, 2015. https://ro.uow.edu.au/thsci/113 Research Online is the open access institutional repository for the University of Wollongong. -

<I>Abralia</I> (Cephalopoda: Enoploteuthidae)
BULLETIN OF MARINE SCIENCE, 66(2): 417–443, 2000 NEW SPECIES PAPER SQUIDS OF THE GENUS ABRALIA (CEPHALOPODA: ENOPLOTEUTHIDAE) FROM THE WESTERN TROPICAL PACIFIC WITH A DESCRIPTION OF ABRALIA OMIAE, A NEW SPECIES Kiyotaka Hidaka and Tsunemi Kubodera ABSTRACT Squids of the genus Abralia collected by a midwater trawl in the western tropical Pa- cific were examined. Five species were identified, including Abralia omiae new species. This species is characterized by having five monotypic subocular photophores, two lon- gitudinal broad bands of minute photophores on the ventral mantle, and a hectocotylized right ventral arm with two crests. The other four species identified were A. similis, A. trigonura, A. siedleckyi and A. heminuchalis. Materials from new localities for these spe- cies are discussed and comparisons to related species in several additional characters are made. Squids of the genus Abralia Gray 1849, family Enoploteuthidae, are important mem- bers in the micronektonic communities in the tropical and subtropical world oceans (e.g., Reid et al., 1991). The genus is distinguished in the family Enoploteuthidae by manus of club with one row of hooks and two rows of suckers, absence of enlarged photophores with black coverage on tips of arm IV, buccal crown with typical chromatophores on aboral surface without any other pigmentation, and five to 12 photophores on eyeball (Young et al., 1998). Several systematic studies on pelagic cephalopods in the Pacific Ocean (Quoy and Gaimard, 1832; Berry, 1909, 1913, 1914; Sasaki, 1929; Grimpe, 1931; Nesis and Nikitina, 1987; Okutani and Tsuchiya, 1987; Burgess, 1992) have revealed that nine nominal Abralia species are known from the Pacific. -

Distribution Patterns of the Early Life Stages of Pelagic Cephalopods in Three Geographically Different Regions of the Arabian Sea
Reprinted from Okutani, T., O'Dor, R.K. and Kubodera, T. (eds.) 1993. Recent Advances in Fisheries Biology (fokai University Press, Tokyo) pp. 417-431. Distribution Patterns of the Early Life Stages of Pelagic Cephalopods in Three Geographically Different Regions of the Arabian Sea Uwe PIATKOWSKI*, Wolfgang WELSCH* and Andreas ROPKE** *Institut fiir Meereskunde, Universitiit Kiel, Diisternbrooker Weg 20, D-2300 Kiel 1, Germany **Institut Jiir Hydrobiologie und Fischereiwissenschaft, Universitiit Hamburg, Olbersweg 24, D-2000 Hamburg 50, Germany Abstract: The present study describes the distribution patterns of the early life stages of pelagic cephalo pods in three different areas of the Arabian Sea, Indian Ocean. Specimens were collected during the Meteor-expedition to the Indian Ocean in 1987 by means of multiple opening/closing nets in the top 150m of the water column. A total of 3836 specimens were caught at 67 stations. The following taxa were prevailing: Sthenoteuthis oualaniensis (Ommastrephidae), Abralia marisarabica and Abraliopsis lineata (Enoploteuthidae), Onychoteuthis banksi (Onychoteuthidae), and Liocranchia reinhardti (Cran chiidae). While the enoploteuthid species dominated the two neritic regions (the stations grids off Oman and Pakistan), the ommastrephid and cranchiid species were most abundant in the oceanic waters of the central Arabian Sea. The geographical and vertical distribution patterns of the taxa were analyzed and are discussed along with hydro graphic features which characterized the different areas. The data provide new and important information on the spawning areas of pelagic tropical cephalopods. Introduction In respect to the strong swimming ability and net-avoidance capability of many adult oceanic cephalo pods, reliable quantitative estimates of their abundances may only be possible through sampling their early life stages. -

Feeding Ecology of Cuvier's Beaked Whale (Ziphius Cavirostris)
J. Mar. Biol. Ass. U.K. 2001), 81,687^694 Printed in the United Kingdom Feeding ecology of Cuvier's beaked whale Ziphius cavirostris): a review with new information on the diet of this species M.B. Santos*, G.J. Pierce*, J. HermanO,A.Lo¨ pezP,A.GuerraP,E.Mente*andM.R.Clarke *Department of Zoology, University of Aberdeen, Tillydrone Avenue, Aberdeen, AB24 2TZ, Scotland. ODepartment of Geology and Zoology, Royal Museums of Scotland, Chambers Street, Edinburgh, EH11JF, Scotland. P ECOBIOMAR, Instituto de Investigaciones Marinas, CSIC, Eduardo Cabello, 6, 36208, Vigo, Pontevedra, Spain. `Ancarva', Southdown, Millbrook, Torpoint, Cornwall, PL10 1EZ. E-mail: [email protected] Published information on the diet of Cuvier's beaked whales Ziphius cavirostris Odontoceti: Ziphiidae) is reviewed and new information on the stomach contents of three animals: two stranded in Galicia north-west Spain) in February 1990 at A Lanzada, and in February 1995 at Portonovo; and the third stranded in February 1999 in North Uist Scotland), is presented. The whale stranded in 1990 was a male; the other two were adult females. All animals were 45 m long. The limited published information on the diet of this species indicates that it feeds primarily on oceanic cephalopods although some authors also found remains of oceanic ¢sh and crustaceans. Food remains from the three new samples consisted entirely of cephalopod beaks. The Scottish sample set is the largest recorded to date for this species. The prey identi¢ed consisted of oceanic cephalopods, mainly squid Cephalopoda: Teuthoidea). The most frequently occurring species were the squid Teuthowenia megalops, Mastigoteuthis schmidti and Taonius pavo for the Galician whale stranded in 1990), Teuthowenia megalops and Histioteuthis reversa for the second Galician whale) and T. -

Cephalopoda: Teuthoidea: Enoploteuthidae
EARLY LIFE HISTORY STAGES OF ENOPLOTEUTHIN SQUIDS (CEPHALOPODA : TEUTHOIDEA : ENOPLOTEUTHIDAE) FROM HAWAIIAN WATERS R Young, R Harman To cite this version: R Young, R Harman. EARLY LIFE HISTORY STAGES OF ENOPLOTEUTHIN SQUIDS (CEPHALOPODA : TEUTHOIDEA : ENOPLOTEUTHIDAE) FROM HAWAIIAN WATERS. Vie et Milieu / Life & Environment, Observatoire Océanologique - Laboratoire Arago, 1985, pp.181-201. hal-03022097 HAL Id: hal-03022097 https://hal.sorbonne-universite.fr/hal-03022097 Submitted on 24 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. VIE MILIEU, 1985, 35 (3/4) : 181-201 EARLY LIFE HISTORY STAGES OF ENOPLOTEUTHIN SQUIDS (CEPHALOPODA : TEUTHOIDEA : ENOPLOTEUTHIDAE) FROM HAWAIIAN WATERS R.E. YOUNG and R.F. HARMAN Department of Oceanography, University of Hawaii, 1000 Pope Road, Honolulu, Hawaii 96822 USA HAWAII ABSTRACT. — Species of the enoploteuthid subfamily Enoploteuthinae spawn LARVAE individual eggs in the plankton. Eggs captured off Hawaii were reared in the CEPHALOPODA laboratory for several days after hatching. The hatchings were matched to size-series ENOPLOTEUTHIDAE of larvae taken from an extensive trawling program designed to catch squid larvae. SQUIDS The early life history stages of thèse species are described and systematic characters VERTICAL-DISTRIBUTION evaluated. -

Diversity of Midwater Cephalopods in the Northern Gulf of Mexico: Comparison of Two Collecting Methods
Diversity of midwater cephalopods in the northern Gulf of Mexico: comparison of two collecting methods H. Judkins, M. Vecchione, A. Cook & T. Sutton Marine Biodiversity ISSN 1867-1616 Mar Biodiv DOI 10.1007/s12526-016-0597-8 1 23 Your article is protected by copyright and all rights are held exclusively by Senckenberg Gesellschaft für Naturforschung and Springer- Verlag Berlin Heidelberg. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Mar Biodiv DOI 10.1007/s12526-016-0597-8 RECENT ADVANCES IN KNOWLEDGE OF CEPHALOPOD BIODIVERSITY Diversity of midwater cephalopods in the northern Gulf of Mexico: comparison of two collecting methods H. Judkins1 & M. Vecchione2 & A. Cook3 & T. Sutton 3 Received: 19 April 2016 /Revised: 28 September 2016 /Accepted: 12 October 2016 # Senckenberg Gesellschaft für Naturforschung and Springer-Verlag Berlin Heidelberg 2016 Abstract The Deepwater Horizon Oil Spill (DWHOS) ne- possible differences in inferred diversity and relative abun- cessitated a whole-water-column approach for assessment that dance. More than twice as many specimens were collected included the epipelagic (0–200 m), mesopelagic (200– with the LMTs than the MOC10, but the numbers of species 1000 m), and bathypelagic (>1000 m) biomes. -

Development of the Ommastrephid Squid Todarodes Pacificus, from Fertilized Egg to the Rhynchoteuthion Paralarva
Title Development of the ommastrephid squid Todarodes pacificus, from fertilized egg to the rhynchoteuthion paralarva Author(s) Watanabe, Kumi; Sakurai, Yasunori; Segawa, Susumu; Okutani, Takashi Citation American Malacological Bulletin, 13(1/2), 73-88 Issue Date 1996 Doc URL http://hdl.handle.net/2115/35243 Type article File Information sakurai-23.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Development of the ommastrephid squid Todarodes pacific us , from fertilized egg to rhynchoteuthion paralarva Kunli Watanabel , Yasunori Sakurai2, Susumu Segawal , and Takashi Okutani3 lLaboratory of Invertebrate Zoology, Tokyo University of Fisheries, Konan, Minato-ku, Tokyo 108, Japan 2Faculty of Fisheries, Hokkaido University, Minatocho, Hakodate, Hokkaido 041, Japan 3College of Bioresource Sciences, Nihon University Fujisawa City, Kanagawa 252, Japan Abstract: The present study establishes for the first time an atlas for the normal development of Todarodes pacificus Steenstrup, 1880, from fertilized egg to rhynchoteuthion paralarva. In the course of the study, observations on embryogenesis and histological differentiation in T. pacificus were made for consideration of the developmental mode of the Oegopsida, which is a specialized group with a reduced external yolk sac. It appears that differentiation of the respiratory and digestive organs is relatively delayed in the Oegopsida, with reduction of the yolk sac as well as the egg size. These characters could be related to a reproductive strategy -
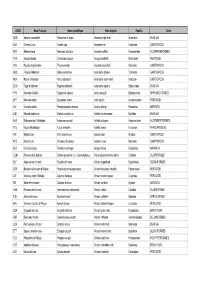
Liste Espèces
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre ODQ Anomie cascabelle Pododesmus cepio Abalone jingle shell Anomiidae BIVALVIA ABX Ormeaux nca Haliotis spp Abalones nei Haliotidae GASTROPODA REN Sébaste rose Sebastes fasciatus Acadian redfish Scorpaenidae SCORPAENIFORMES YNA Acoupa toeroe Cynoscion acoupa Acoupa weakfish Sciaenidae PERCOIDEI HSV Pourpre aiguillonnee Thais aculeata Aculeate rock shell Muricidae GASTROPODA GBQ Troque d'Adanson Gibbula adansoni Adanson's gibbula Trochidae GASTROPODA NKA Natice d'Adanson Natica adansoni Adanson's moon snail Naticidae GASTROPODA GLW Tagal d'Adanson Tagelus adansonii Adanson's tagelus Solecurtidae BIVALVIA PYD Manchot d'Adélie Pygoscelis adeliae Adelie penguin Spheniscidae SPHENISCIFORMES QFT Maconde aden Synagrops adeni Aden splitfin Acropomatidae PERCOIDEI NIV Crevette adonis Parapenaeopsis venusta Adonis shrimp Penaeidae NATANTIA DJD Modiole adriatique Modiolus adriaticus Adriatic horse mussel Mytilidae BIVALVIA AAA Esturgeon de l'Adriatique Acipenser naccarii Adriatic sturgeon Acipenseridae ACIPENSERIFORMES FCV Fucus d'Adriatique Fucus virsoides Adriatic wrack Fucaceae PHAEOPHYCEAE IRR Mitre brûlée Mitra eremitarum Adusta miter Mitridae GASTROPODA KCE Murex bruni Chicoreus brunneus Adusta murex Muricidae GASTROPODA AES Crevette ésope Pandalus montagui Aesop shrimp Pandalidae NATANTIA CGM Poisson-chat, hybride Clarias gariepinus x C. macrocephalus Africa-bighead catfish, hybrid Clariidae SILURIFORMES SUF Ange de mer africain Squatina africana African angelshark Squatinidae SQUALIFORMES -

Analyzing Pelagic Food Webs Leading to Top Predators in the Pacific Ocean
Progress in Oceanography 86 (2010) 152–165 Contents lists available at ScienceDirect Progress in Oceanography journal homepage: www.elsevier.com/locate/pocean Analyzing pelagic food webs leading to top predators in the Pacific Ocean: A graph-theoretic approach Jeffrey M. Dambacher a,*, Jock W. Young b, Robert J. Olson c, Valérie Allain d, Felipe Galván-Magaña e, Matthew J. Lansdell b, Noemí Bocanegra-Castillo e, Vanessa Alatorre-Ramírez e, Scott P. Cooper b, Leanne M. Duffy c a CSIRO Mathematics, Informatics and Statistics, GPO Box 1538, Hobart, Tasmania 7001, Australia b CSIRO Marine and Atmospheric Research, GPO Box 1538, Hobart, Tasmania 7001, Australia c Inter-American Tropical Tuna Commission, 8604 La Jolla Shores Drive, La Jolla, CA 92037, USA d Secretariat of the Pacific Community, BP D5, 98848 Noumea, New Caledonia e Centro Interdisciplinario de Ciencias Marinas, Instituto Politécnico Nacional, Apartado Postal 592, La Paz, Baja California Sur, C.P. 23000, Mexico article info abstract Article history: This work examined diet data from studies of top pelagic predators from three large regions of the equa- Received 14 March 2008 torial and South Pacific Ocean. Food webs of each of these three systems were found to have relatively Received in revised form 16 June 2009 high species diversity, but in contrast to other marine systems, relatively low connectance. Food webs Accepted 10 April 2010 were examined using graph-theoretic methods, which included aggregating species based on food-web Available online 20 April 2010 relationships and identification of potentially influential species. Species aggregations were used to con- struct simplified qualitative models from each region’s food web. -

FAU Institutional Repository
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©1992 Springer. This manuscript is an author version with the final publication available at http://www.springerlink.com and may be cited as: Herring, P. J., Widder, E. A., & Haddock, S. H. D. (1992). Correlation of bioluminescence emissions with ventral photophores in the mesopelagic squid Abralia veranyi (Cephalopoda: Enoploteuthidae). Marine Biology, 112(2), 293‐298. doi:10.1007/BF00702474 I I Marine Biology 112, 293- 298 (1992) Marine lntemattonat dournal llfSB~~7/ In on life Oceans B-Iology J--_--- () and Coastal Waters JJ-)P\J~ - © Springer-Verlag 1992 J Correlation of bioluminescence emissions with ventral photophores in the mesopelagic squid Ahralia veranyi (Cephalopoda: Enoploteuthidae) P. J. Herring I, E. A. Widder 2 and S. H. D. Haddock 3 1 Institute of Oceanographic Sciences, Deacon Laboratory, Brook Road, Wormley, Godalming GU8 5UB, Surrey, England 2 Harbor Branch Oceanographic Institution, 5600 Old Dixie Highway, Fort Pierce, Florida 34946, USA 3 Marine Science Institute, University of California, Santa Barbara, California 93106, USA Date of final manuscript acceptance: September 26, 1991. Communicated by 1. Mauchline, Oban Abstract. Specimens of the squid Abralia veranyi were spinosus and Systellaspis debilis is spectrally different collected off the Bahamas in 1989. The bioluminescence from that emitted by the photophores ofthe same species of the ventral photophores was recorded on videotape at (Herring 1983, Latz et al. 1988), and in many animals the 11 to 12°C and 24 °C, in conjunction with measurements emission spectra are determined, in part, by the effects of of the spectral emission at the same temperatures.