Development of the Ommastrephid Squid Todarodes Pacificus, from Fertilized Egg to the Rhynchoteuthion Paralarva
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
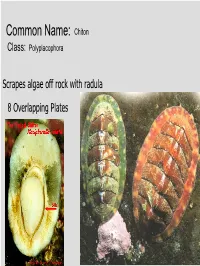
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

The Cephalopoda
Carl Chun THE CEPHALOPO PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Carl Chun THE CEPHALOPODA NOTE TO PLATE LXVIII Figure 7 should read Figure 8 Figure 9 should read Figure 7 GERMAN DEEPSEA EXPEDITION 1898-1899. VOL. XVIII SCIENTIFIC RESULTS QF THE GERMAN DEEPSEA EXPEDITION ON BOARD THE*STEAMSHIP "VALDIVIA" 1898-1899 Volume Eighteen UNDER THE AUSPICES OF THE GERMAN MINISTRY OF THE INTERIOR Supervised by CARL CHUN, Director of the Expedition Professor of Zoology , Leipzig. After 1914 continued by AUGUST BRAUER Professor of Zoology, Berlin Carl Chun THE CEPHALOPODA PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Translatedfrom the German ISRAEL PROGRAM FOR SCIENTIFIC TRANSLATIONS Jerusalem 1975 TT 69-55057/2 Published Pursuant to an Agreement with THE SMITHSONIAN INSTITUTION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D.C. Since the study of the Cephalopoda is a very specialized field with a unique and specific terminology and phrase- ology, it was necessary to edit the translation in a technical sense to insure that as accurate and meaningful a represen- tation of Chun's original work as possible would be achieved. We hope to have accomplished this responsibility. Clyde F. E. Roper and Ingrid H. Roper Technical Editors Copyright © 1975 Keter Publishing House Jerusalem Ltd. IPST Cat. No. 05452 8 ISBN 7065 1260 X Translated by Albert Mercado Edited by Prof. O. Theodor Copy-edited by Ora Ashdit Composed, Printed and Bound by Keterpress Enterprises, Jerusalem, Israel Available from the U. S. DEPARTMENT OF COMMERCE National Technical Information Service Springfield, Va. 22151 List of Plates I Thaumatolampas diadema of luminous o.rgans 95 luminous organ 145 n.gen.n.sp. -

Biodiversity of Cephalopod Early-Life Stages Across the Southeastern Brazilian Bight: Spatio-Temporal Patterns in Taxonomic Richness
Marine Biodiversity https://doi.org/10.1007/s12526-019-00980-w ORIGINAL PAPER Biodiversity of cephalopod early-life stages across the Southeastern Brazilian Bight: spatio-temporal patterns in taxonomic richness Carolina C. Araújo1,2 & Maria A. Gasalla1,2 Received: 15 October 2018 /Revised: 20 May 2019 /Accepted: 5 June 2019 # Senckenberg Gesellschaft für Naturforschung 2019 Abstract The diversity patterns of cephalopod early-life stages on the continental shelf of Southeastern Brazilian Bight (SBB, 22–25°S) were investigated using a historical plankton archive of 22 oceanographic cruises carried out from 1974 to 2010. From 874 plankton samples, 438 were positive for cephalopod paralarvae (n = 2116), which were identified to the lowest taxonomic level possible, totaling 15 taxa belonging to 11 families. Richness and diversity indexes (Shannon-Wiener, Simpson, Pielou’seven- ness) revealed a cross-shelf gradient, independent of season and latitude. Abundance k-dominance curves were consistent with this depth-related trend, resulting in high values of k-dominance for the inner shelf during both summer and winter. Two major assemblages were identified by cluster analyses: an inner shelf and a mid-outer shelf. During summer, the inner shelf assemblage was composed of neritic Loliginidae Lesueur, 1821 and epipelagic Argonautidae Tryon, 1879, while in winter, benthic Octopodidae Orbigny, 1840 replaced Argonautidae in importance. These data reveal a remarkable difference in Argonautidae and Octopodidae paralarvae abundance, suggesting a seasonal reproductive pattern for these cephalopods in the SBB. Mesopelagic Enoploteuthidae Pfeffer, 1900 and Ommastrephidae Steenstrup, 1857 characterized the mid-outer shelf assem- blages both in summer and winter. Although based on a higher taxonomic level, the distribution of cephalopod paralarva families reflected not only oceanographic patterns of the SBB but also their adaptations and reproductive strategies. -

Learning About Our Favourite Squid Species
Cephalopod Science Investigations LEARNING ABOUT OUR FAVOURITE SQUID SPECIES By Cushla Dromgool-Regan Eimear Manning & Anna Quinn www.EXPLORERS.ie The Explorers Education Programme is funded by the Marine Institute Explorers Education Programme engage with primary schools, teachers and children, creating marine leaders and ocean champions. The Explorers Education Programme team provides engaging activities, resources and support for teachers, children and the education network, delivering ocean literacy to primary schools. We aim to inspire children and educators to learn about our marine and maritime identity and heritage, as well as making informed and responsible decisions regarding the ocean and its resources. We communicate about the ocean in a meaningful way, increasing the awareness and understanding of our marine biodiversity, the environment, as well as the opportunities and social benefits of our ocean wealth. To help inspire children learning about the ocean, we have developed a series of teaching materials and resources about Squid! Check out our Explorers books: Cephalopod Science Investigations – Learning about Squid 101; My CSI Squid Workbook. Also, see our interactive film: Cephalopod Science Investigations – Learning about Squid 101 and Dissection. For more information about our Squid series see www.explorers.ie CEPHALOPOD SCIENCE INVESTIGATIONS LEARNING ABOUT OUR FAVOURITE SQUID SPECIES AUTHORS Cushla Dromgool-Regan Eimear Manning Anna Quinn PUBLISHED BY Marine Institute First published in 2021 Marine Institute, Rinville, Oranmore, Galway All or parts of the content of this publication may be reproduced without further permission for education purposes, provided the author and publisher are acknowledged. Authors: Cushla Dromgool-Regan, The Camden Education Trust; Eimear Manning, The Camden Education Trust; & Anna Quinn, Galway Atlantaquaria. -

Bio-Inspired Artificial Helical Chromatophore for Stealth Mode
Bio-inspired Artificial Helical Chromatophore for Stealth Mode Duck Weon Lee Department of Chemistry and Material Science, Aalto University, PO Box 16100, FI-00076 AALTO, Finland Abstract Stealth technology has been very usefully applied in the military fields and is now becoming more prominent as a strategic technology. In nature, the firefly squid can protect itself from enemies using camouflage as a stealth mode. On the other hand, it is able to send fluorescent signals to attract prey by switching into a bright mode. Despite the development of many existing biomimetic materials, there are significant constraints related to their color-changeable velocity and mobility. Herein, we have developed a bio-inspired artificial thermochromic material system, which can reversibly switch between stealth and bright modes and thus provide a means to adapting to one’s environment analogous to the strategy applied by firefly squids. Through vertical contraction, a helically coiled yarn artificial muscle, selectively coated by Rhodamine B and TiO2, can switch between fluorescent and stealth modes with a maximum speed of 0.31 cm/s. Upon external thermal impulse, artificial thermochromic muscle can spin up to 309° and achieve a negative strain of 84.6%. In addition, this research demonstrates thermochromic effects even in underwater aqueous conditions, showing applicability toward underwater robotics. With the cost-effectiveness of the demonstrated system, the developed artificial thermochromic muscles can be implemented into a variety of applications, such as colorimetric sensors and aqueous color-changeable soft robotics. Stealth technology, a type of camouflage, can be used as an antidetection technology to hide aircraft, submarines, satellites, and missiles from sonar, infrared, radar, and other detectors which take advantage of a power and direction of electromagnetic radiation1–3. -

Husbandry Manual for BLUE-RINGED OCTOPUS Hapalochlaena Lunulata (Mollusca: Octopodidae)
Husbandry Manual for BLUE-RINGED OCTOPUS Hapalochlaena lunulata (Mollusca: Octopodidae) Date By From Version 2005 Leanne Hayter Ultimo TAFE v 1 T A B L E O F C O N T E N T S 1 PREFACE ................................................................................................................................ 5 2 INTRODUCTION ...................................................................................................................... 6 2.1 CLASSIFICATION .............................................................................................................................. 8 2.2 GENERAL FEATURES ....................................................................................................................... 8 2.3 HISTORY IN CAPTIVITY ..................................................................................................................... 9 2.4 EDUCATION ..................................................................................................................................... 9 2.5 CONSERVATION & RESEARCH ........................................................................................................ 10 3 TAXONOMY ............................................................................................................................12 3.1 NOMENCLATURE ........................................................................................................................... 12 3.2 OTHER SPECIES ........................................................................................................................... -

Giant Pacific Octopus (Enteroctopus Dofleini) Care Manual
Giant Pacific Octopus Insert Photo within this space (Enteroctopus dofleini) Care Manual CREATED BY AZA Aquatic Invertebrate Taxonomic Advisory Group IN ASSOCIATION WITH AZA Animal Welfare Committee Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Aquatic Invertebrate Taxon Advisory Group (AITAG) (2014). Giant Pacific Octopus (Enteroctopus dofleini) Care Manual. Association of Zoos and Aquariums, Silver Spring, MD. Original Completion Date: September 2014 Dedication: This work is dedicated to the memory of Roland C. Anderson, who passed away suddenly before its completion. No one person is more responsible for advancing and elevating the state of husbandry of this species, and we hope his lifelong body of work will inspire the next generation of aquarists towards the same ideals. Authors and Significant Contributors: Barrett L. Christie, The Dallas Zoo and Children’s Aquarium at Fair Park, AITAG Steering Committee Alan Peters, Smithsonian Institution, National Zoological Park, AITAG Steering Committee Gregory J. Barord, City University of New York, AITAG Advisor Mark J. Rehling, Cleveland Metroparks Zoo Roland C. Anderson, PhD Reviewers: Mike Brittsan, Columbus Zoo and Aquarium Paula Carlson, Dallas World Aquarium Marie Collins, Sea Life Aquarium Carlsbad David DeNardo, New York Aquarium Joshua Frey Sr., Downtown Aquarium Houston Jay Hemdal, Toledo -

Octopus Insularis</Italic> As a New Marine Model for Evolutionary
© 2019. Published by The Company of Biologists Ltd | Biology Open (2019) 8, bio046086. doi:10.1242/bio.046086 RESEARCH ARTICLE Octopus insularis as a new marine model for evolutionary developmental biology Ernesto Maldonado1,*, Emma Rangel-Huerta1,2, Roberto González-Gómez3,4, Gabriel Fajardo-Alvarado3,4 and Piedad S. Morillo-Velarde4,5,* ABSTRACT of aquatic animal eggs and embryos guarantees the observation of Octopuses are intriguing organisms that, together with squids and every developmental stage using microscopy and allows detailed cuttlefishes, form the extant coleoid cephalopods. This group includes experimental analysis from the first cell division through to the many species that can potentially be used as models in the fields of formation of embryonic germ layers and organogenesis (Boletzky biomedicine, developmental biology, evolution, neuroscience and et al., 2006). Finally, small embryos allow reasonable sample sizes even for robotics research. The purpose of this work is to first to be tested together using multi-well plates to provide multiple present a simple method for maintaining Octopus insularis embryos experimental replicates at the same time, making them cost- under a laboratory setup. Second, we show that these embryos are effective animal models (Hill et al., 2005). suitable for detailed analyses of specific traits that appear during Coleoid cephalopods (octopus, squid and cuttlefish) exhibit the developmental stages, including the eyes, hearts, arms, suckers, largest nervous systems found among invertebrates (Young, 1971) chromatophores and Kölliker’s organs. Similar complex traits between and a sophisticated visual system controlling body color changes for cephalopods and vertebrates such as the visual, cardiovascular, communication, camouflage and mimicry (Hanlon et al., 2011; neural and pigmentation systems are generally considered to be a Robin et al., 2014). -

Estimation of Biomass, Production and Fishery Potential of Ommastrephid Squids in the World Ocean and Problems of Their Fishery Forecasting
ICES CM 2004 / CC: 06 ESTIMATION OF BIOMASS, PRODUCTION AND FISHERY POTENTIAL OF OMMASTREPHID SQUIDS IN THE WORLD OCEAN AND PROBLEMS OF THEIR FISHERY FORECASTING Ch. M. Nigmatullin Atlantic Research Institute of Marine Fisheries and Oceanography (AtlantNIRO), Dm. Donskoj Str. 5, Kaliningrad, 236000 Russia [tel. +0112-225885, fax + 0112-219997, e-mail: [email protected]] ABSTRACT 21 species of the nektonic squids family Ommastrephidae inhabits almost the entire waters of the World Ocean. It is the most commercial important group among cephalopods. Straight and expert evaluations of biomass were carried out for each species. In all ommastrephids the total instantaneous biomass is ~55 million t on average and total yearly production is ~ 400 million t (production/biomass coefficient - P/B = 5 in inshore species and 8 - in oceanic ones). Now there are 12-fished species, mainly 8 inshore ones. In 1984-2001 the yearly world catch of ommastrephids was about 1.5-2.2 million t (=50-65% of total cephalopod catch). The feasible ommastrephids fishery potential is ~ 6-9 million t including 4-7 million t of oceanic species. Thus ommastrephids are one of the most important resources for increasing high-quality food protein catch in the World Ocean. At the same time there are serious economical and technical difficulties to develop oceanic resources fishery, especially for Ommastrephes and Sthenoteuthis. A general obstacle in the real fishery operations and fishery forecasting for ommastrephids is their r-strategist ecological traits, related to monocyclia, short one-year life cycle, pelagic egg masses, paralarvae and fry, and accordingly high mortality rate during two last ontogenetic stages. -

Ommastrephidae 199
click for previous page Decapodiformes: Ommastrephidae 199 OMMASTREPHIDAE Flying squids iagnostic characters: Medium- to Dlarge-sized squids. Funnel locking appara- tus with a T-shaped groove. Paralarvae with fused tentacles. Arms with biserial suckers. Four rows of suckers on tentacular clubs (club dactylus with 8 sucker series in Illex). Hooks never present hooks never on arms or clubs. One of the ventral pair of arms present usually hectocotylized in males. Buccal connec- tives attach to dorsal borders of ventral arms. Gladius distinctive, slender. funnel locking apparatus with Habitat, biology, and fisheries: Oceanic and T-shaped groove neritic. This is one of the most widely distributed and conspicuous families of squids in the world. Most species are exploited commercially. Todarodes pacificus makes up the bulk of the squid landings in Japan (up to 600 000 t annually) and may comprise at least 1/2 the annual world catch of cephalopods.In various parts of the West- ern Central Atlantic, 6 species of ommastrephids currently are fished commercially or for bait, or have a potential for exploitation. Ommastrephids are powerful swimmers and some species form large schools. Some neritic species exhibit strong seasonal migrations, wherein they occur in huge numbers in inshore waters where they are accessable to fisheries activities. The large size of most species (commonly 30 to 50 cm total length and up to 120 cm total length) and the heavily mus- cled structure, make them ideal for human con- ventral view sumption. Similar families occurring in the area Onychoteuthidae: tentacular clubs with claw-like hooks; funnel locking apparatus a simple, straight groove. -

Description of Ommastrephes Bartramii (Cephalopoda: Ommastrephidae) Paralarvae with Evidence for Spawning in Hawaiian Waters!
Pacific Science (1990), vol. 44, no. 1: 71-80 © 1990 by University of Hawaii Press. All rights reserved Description of Ommastrephes bartramii (Cephalopoda: Ommastrephidae) Paralarvae with Evidence for Spawning in Hawaiian Waters! RICHARD EDWARD YOUNG AND JED HIROTA2 ABSTRACT: Paralarvae of the commercially important squid Ommastrephes bartramii are described, and particular attention is paid to the chromatophore patterns. These chromatophore patterns are compared with those ofStenoteuthis oualaniensis and Hyaloteuthis pelagica , two other local ommastrephids with similar patterns. Limited data on spatial and temporal distributions of the paralarvae are also presented. Paralarvae of O. bartramii have been found at several localities along the Hawaiian Archipelago between Oahu and Midway Islands. In 1986 O. bartramii probably spawned in southern Hawaiian waters around the island of Oahu from, at least, the latter part of February through March. During April 1979 and April 1984 the absence ofparalarvae from these same waters suggests that either the spawning period terminated earlier or the squid did not spawn as far south as in 1986. High abundances of O. bartramii paralarvae in some April 1979 samples suggest that spawning was more intense in the northwestern half of the Hawaiian Archipelago. Ommastrephes bartramii is broadly distributed the possibility ofthree spawning regions in the throughout the subtropical and temperate North Pacific: one at ca. 140- 150° E long., waters of the world's oceans. In the Pacific the one at ca. 170° E long., and one between 160 northern and southern populations are dis 180° W long. Additional evidence for the first continuous (Wormuth 1976). The North Pa site has been provided by Nakamura (1988) cific population is spread acro ss the open based on the distribution of mature females ocean, where it is fished with jigs and drift nets and by Okutani (1968) based on the presence over much of its range . -

1. in Tro Duc Tion
Cephalopods of the World 1 1. INTRO DUC TION Patrizia Jereb, Clyde F.E. Roper and Michael Vecchione he increasing exploitation of finfish resources, and the commercial status. For example, this work should be useful Tdepletion of a number of major fish stocks that formerly for the ever-expanding search for development and supported industrial-scale fisheries, forces continued utilization of ‘natural products’, pharmaceuticals, etc. attention to the once-called ‘unconventional marine resources’, which include numerous species of cephalopods. The catalogue is based primarily on information available in Cephalopod catches have increased steadily in the last 40 published literature. However, yet-to-be-published reports years, from about 1 million metric tonnes in 1970 to more than and working documents also have been used when 4 million metric tonnes in 2007 (FAO, 2009). This increase appropriate, especially from geographical areas where a confirms a potential development of the fishery predicted by large body of published information and data are lacking. G.L. Voss in 1973, in his first general review of the world’s We are particularly grateful to colleagues worldwide who cephalopod resources prepared for FAO. The rapid have supplied us with fisheries information, as well as expansion of cephalopod fisheries in the decade or so bibliographies of local cephalopod literature. following the publication of Voss’s review, meant that a more comprehensive and updated compilation was required, The fishery data reported herein are taken from the FAO particularly for cephalopod fishery biologists, zoologists and official database, now available on the Worldwide web: students. The FAO Species Catalogue, ‘Cephalopods of the FISHSTAT Plus 2009.