Octopus Insularis</Italic> As a New Marine Model for Evolutionary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
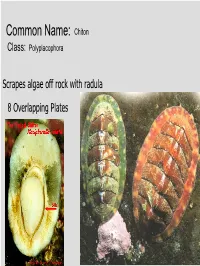
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Cephalopoda: Octopodidae): the Smallest Southwestern Atlantic Octopod, Found in Sea Debris
A new species of pygmy Paroctopus (Cephalopoda: Octopodidae): the smallest southwestern Atlantic octopod, found in sea debris Tatiana S. Leite ( [email protected] ) Universidade Federal de Santa Catarina Centro de Ciencias Biologicas https://orcid.org/0000-0001-9117-9648 Erica A.G. Vidal Universidade Federal do Parana Setor de Ciencias da Terra Françoise Dantas Lima Universidade Federal do Rio Grande do Norte Centro de Biociencias Sergio M.Q. Lima Universidade Federal do Rio Grande do Norte Centro de Biociencias Ricardo M Dias Universidade Federal do Sul da Bahia Giulia A. Giuberti Universidade Federal do Estado do Rio de Janeiro Davi De Vasconcellos Universidade Federal do Rio Grande Jennifer A. Mather University of Lethbridge Manuel Haimovici Universidade Federal do Rio Grande Original Paper Keywords: Paroctopus, octopus Posted Date: January 29th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-172910/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Marine Biodiversity on July 27th, 2021. See the published version at https://doi.org/10.1007/s12526-021-01201-z. Loading [MathJax]/jax/output/CommonHTML/fonts/TeX/fontdata.js Page 1/27 Abstract The new species, Paroctopus cthulu sp. nov. Leite, Haimovici, Lima and Lima, was recorded from very shallow coastal waters on sandy/muddy and shelter- poor bottoms with natural and human-origin debris. It is a small octopus, adults are less than 35 mm mantle length (ML) and weigh around 15 g. It has short to medium sized arms, enlarged suckers on the arms of both males and females, large posterior salivary glands (25 %ML), a relatively large beak (9 % ML) and medium to large mature eggs (3.5 to > 9 mm). -

Spaceflight Imposes Numerous Adaptive Challenges for Terrestrial Life
Astrobiology Science Conference 2017 (LPI Contrib. No. 1965) 3032.pdf Transcriptomic changes in an animal-bacterial symbiosis under modeled microgravity conditions. Giorgio Casaburi1, Irina Goncharenko-Foster1 and Jamie S. Foster1, 1Department of Microbiology and Cell Science, University of Florida, Space Life Science Lab, Merritt Island, FL, USA. Introduction: Spaceflight imposes numerous adaptive challenges for terrestrial life. The reduction in gravity, or microgravity, represents a novel environ- ment that can disrupt homeostasis of many physiologi- cal processes. Additionally, it is becoming increasingly clear that an organism’s microbiome is critical for host health and examining its resiliency in microgravity represents a new frontier for space biology research. In this study, we examine the impact of microgravity on the interactions between the squid Euprymna scolopes and its beneficial symbiont Vibrio fischeri, which form a highly specific binary mutualism. First, animals in- oculated with V. fischeri aboard the space shuttle showed effective colonization of the host light organ, the site of the symbiosis, during space flight. Second, RNA-Seq analysis of squid exposed to modeled mi- crogravity conditions exhibited extensive differential gene expression in the presence and absence of the symbiotic partner. Transcriptomic analyses revealed in the absence of the symbiont during modeled micro- gravity there was an enrichment of genes and pathways associated with the innate immune and oxidative stress response. The results suggest that V. fischeri may help modulate the host stress responses under modeled mi- crogravity. This study provides a window into the adaptive responses that the host animal and its symbi- ont use during modeled microgravity. . -

Counterillumination in the Hawaiian Bobtail Squid, Euprymna Scolopes Berry (Mollusca: Cephalopoda)
Marine Biology (2004) 144: 1151–1155 DOI 10.1007/s00227-003-1285-3 RESEARCH ARTICLE B. W. Jones Æ M. K. Nishiguchi Counterillumination in the Hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda) Received: 27 May 2003 / Accepted: 24 November 2003 / Published online: 10 January 2004 Ó Springer-Verlag 2004 Abstract The mutualism between the Hawaiian bobtail 1999), predator evasion (Hartline et al. 1999), and squid Euprymna scolopes and the luminescent symbiont counterillumination, an antipredatory behavior com- Vibrio fischeri has been used extensively as a model mon to many midwater cephalopods, decapod crusta- system for studies ranging from co-speciation and bio- ceans, and fishes (Young 1977; Harper and Case 1999; geography to gene regulation and the evolution of Lindsay et al. 1999). Animals exhibiting counterillumi- pathogenesis. In this association, the luminescent bac- nation reduce their silhouette by producing biolumi- terium V. fischeri is housed in a complex light organ nescence in an attempt to match the intensity and within the mantle cavity of E. scolopes. Prior hypotheses wavelength of down-welling light (Young and Roper have assumed that sepiolid squids in general utilize the 1977), providing a mechanism that allows them to evade bioluminescence produced by their V. fischeri symbionts predators by camouflage. The light produced can either for counterillumination, a behavior that helps squid be autogenic (luminescence produced intrinsically by the camouflage themselves by matching down-welling animal itself), or bacteriogenic (produced by bacterial moonlight via silhouette reduction. This assumption, symbionts). based solely on the morphology of the squid light organ, Establishing a morphological design for efficient has never been empirically tested for Euprymna in the counterillumination has resulted in the evolution of a laboratory. -

Glossary for Hawaiian and Other Polynesian Terms
Glossary for Hawaiian and Other Polynesian Terms Pronunciation Hawaiian vowels are as in English: a, e, i, o, and u. But with respect to pronunciation, the letter “a” is pronounced as the soft ah sound in papa; “e” as the ā sound in play; “i” as the ē sound in need; “o” as used in bowl; and “u” as the ew sound in tune. Diacritical marks are used to indicate stress on particular vowels, and as glottal stops. Te macron (called kahakō in Hawaiian) is used to stress and elongate any of the vowel sounds. For example, the ā sound in pāhoe- hoe (sheet lava) is stressed and lengthened, as in pahh-ho-ay-ho-ay. Te reverse apostrophe (called an okina in Hawaiian) is used as a glot- tal stop, as in the closed throat sound that should precede formation of the word ‘ahi (pronounced ah-hee), or between sounds, as in Punalu‘u (pronounced poo-nah-lew-ew). Certain vowel combinations (diph- thongs) are also pronounced in a manner dissimilar to the way they are pronounced in English, with stress on the frst vowel. For instance, the “ou” sound in Hawaiian is pronounced with stress on the o, as in pouli © Te Editor(s) (if applicable) and Te Author(s), under exclusive license 247 to Springer Nature Switzerland AG 2019 E. W. Glazier, Tradition-Based Natural Resource Management, Palgrave Studies in Natural Resource Management, https://doi.org/10.1007/978-3-030-14842-3 248 Glossary for Hawaiian and Other Polynesian Terms (Hawaiian for dark or eclipse, pronounced poh-lee). -

Geographic Variability of Octopus Insularis Diet: from Oceanic Island to Continental Populations
Vol. 25: 17–27, 2016 AQUATIC BIOLOGY Published June 14 doi: 10.3354/ab00655 Aquat Biol OPENPEN ACCESSCCESS Geographic variability of Octopus insularis diet: from oceanic island to continental populations Tatiana S. Leite1,*, Allan T. Batista2, Françoise D. Lima2, Jaciana C. Barbosa1, Jennifer Mather3 1Department of Oceanography and Limnology, Federal University of Rio Grande do Norte, Via Costeira, CEP 59014-100 Natal, RN, Brazil 2Ecology Pos-Graduation, Federal University of Rio Grande do Norte, Via Costeira, CEP 59014-100 Natal, RN, Brazil 3Psychology Department, University of Lethbridge, Lethbridge AB T1K 3M4, Canada ABSTRACT: A predator’s choice of prey can be affected by many factors. We evaluated various influences on population dietary composition, individual specialization and size of prey in Octopus insularis populations from 2 continental and 4 insular locations. We expected that habitat diversity would lead to diet heterogeneity. Furthermore, in keeping with MacArthur & Wilson’s (1967) the- ory of island biogeography, we expected that diet diversity would be lower around islands than on the coast of the mainland. Both predictions were confirmed when prey remains from octopus mid- dens were examined. The 2 continental areas exhibited a richer habitat diversity and a wider vari- ety of prey. Niche widths in the continental areas were 2.42 and 2.03, with the lowest niche widths exhibited by the population from the most distant oceanic islands (1.30, 0.85). We found variation in the proportion of specialist relative to generalist individuals across areas based on the propor- tional similarity index. The correlation between habitat diversity and niche width (R2 = 0.84) was highly significant, as was distance from the continental shelf and niche width (R2 = 0.89). -

1. in Tro Duc Tion
Cephalopods of the World 1 1. INTRO DUC TION Patrizia Jereb, Clyde F.E. Roper and Michael Vecchione he increasing exploitation of finfish resources, and the commercial status. For example, this work should be useful Tdepletion of a number of major fish stocks that formerly for the ever-expanding search for development and supported industrial-scale fisheries, forces continued utilization of ‘natural products’, pharmaceuticals, etc. attention to the once-called ‘unconventional marine resources’, which include numerous species of cephalopods. The catalogue is based primarily on information available in Cephalopod catches have increased steadily in the last 40 published literature. However, yet-to-be-published reports years, from about 1 million metric tonnes in 1970 to more than and working documents also have been used when 4 million metric tonnes in 2007 (FAO, 2009). This increase appropriate, especially from geographical areas where a confirms a potential development of the fishery predicted by large body of published information and data are lacking. G.L. Voss in 1973, in his first general review of the world’s We are particularly grateful to colleagues worldwide who cephalopod resources prepared for FAO. The rapid have supplied us with fisheries information, as well as expansion of cephalopod fisheries in the decade or so bibliographies of local cephalopod literature. following the publication of Voss’s review, meant that a more comprehensive and updated compilation was required, The fishery data reported herein are taken from the FAO particularly for cephalopod fishery biologists, zoologists and official database, now available on the Worldwide web: students. The FAO Species Catalogue, ‘Cephalopods of the FISHSTAT Plus 2009. -

Lab 5: Phylum Mollusca
Biology 18 Spring, 2008 Lab 5: Phylum Mollusca Objectives: Understand the taxonomic relationships and major features of mollusks Learn the external and internal anatomy of the clam and squid Understand the major advantages and limitations of the exoskeletons of mollusks in relation to the hydrostatic skeletons of worms and the endoskeletons of vertebrates, which you will examine later in the semester Textbook Reading: pp. 700-702, 1016, 1020 & 1021 (Figure 47.22), 943-944, 978-979, 1046 Introduction The phylum Mollusca consists of over 100,000 marine, freshwater, and terrestrial species. Most are familiar to you as food sources: oysters, clams, scallops, and yes, snails, squid and octopods. Some also serve as intermediate hosts for parasitic trematodes, and others (e.g., snails) can be major agricultural pests. Mollusks have many features in common with annelids and arthropods, such as bilateral symmetry, triploblasty, ventral nerve cords, and a coelom. Unlike annelids, mollusks (with one major exception) do not possess a closed circulatory system, but rather have an open circulatory system consisting of a heart and a few vessels that pump blood into coelomic cavities and sinuses (collectively termed the hemocoel). Other distinguishing features of mollusks are: z A large, muscular foot variously modified for locomotion, digging, attachment, and prey capture. z A mantle, a highly modified epidermis that covers and protects the soft body. In most species, the mantle also secretes a shell of calcium carbonate. z A visceral mass housing the internal organs. z A mantle cavity, the space between the mantle and viscera. Gills, when present, are suspended within this cavity. -

Cover Image the Hawaiian Bobtail Squid
SUBSCRIPTIONS In 2020 Phil. Trans. R. Soc. B (ISSN 0962-8436) will be published Continuing its long history of infl uential scientifi c publishing, Phil. Trans. R. Soc. B 26 times a year. For more details of publishes high quality theme issues on topics of current importance and general subscriptions and single issue sales interest within the life sciences, guest-edited by leading authorities and comprising please contact our fulfi lment agent: new research, reviews and opinions from prominent researchers. Each issue Turpin Distribution aims to create an original and authoritative synthesis, often bridging traditional The Royal Society Customer Services Pegasus Drive disciplines, which showcases current developments and provides a foundation for Stratton Business Park future research, applications and policy decisions. Biggleswade SG18 8TQ United Kingdom royalsocietypublishing.org/journal/rstb T +44 1767 604951 F +44 1767 601640 E [email protected] EDITOR Karen Lipkow Alternatively, please contact our customer John Pickett Karen Liu service team at: Satyajit Mayor E [email protected] SENIOR COMMISSIONING EDITOR Ewa Paluch Helen Eaton Ricard Solé PRICES FOR 2020 Roland Wedlich-Söldner PRODUCTION EDITOR Online Online Garrett Ziolek Neuroscience and cognition only and print Dora Biro EDITORIAL BOARD Anna Borghi £ UK/rest of World £2812 £3936 Organismal, environmental Nicole Creanza and evolutionary biology Patricia Pestana Garcez € Europe €3656 €5118 Julia Blanchard Anthony Isles $ US/Canada $5323 $7452 Patrick Butler -

Identification and Gene Expression of Multiple Peptidoglycan Recognition
Identification and gene expression of multiple peptidoglycan recognition proteins (PGRPs) in the deep-sea mussel Bathymodiolus azoricus, involvement in symbiosis? Camille Détrée, François H. Lallier, Arnaud Tanguy, Jean Mary To cite this version: Camille Détrée, François H. Lallier, Arnaud Tanguy, Jean Mary. Identification and gene expression of multiple peptidoglycan recognition proteins (PGRPs) in the deep-sea mussel Bathymodiolus azoricus, involvement in symbiosis?. Comparative Biochemistry and Physiology - Part B: Biochemistry and Molecular Biology, Elsevier, 2017, 207, pp.1-8. 10.1016/j.cbpb.2017.02.002. hal-01469197 HAL Id: hal-01469197 https://hal.sorbonne-universite.fr/hal-01469197 Submitted on 16 Feb 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ACCEPTED MANUSCRIPT Détrée et al. 2017 Identification and gene expression of multiple peptidoglycan recognition proteins (PGRPs) in the deep-sea mussel Bathymodiolus azoricus, involvement in symbiosis? Camille Détrée1,2, François H. Lallier1, Arnaud Tanguy1, Jean Mary1,* 1Sorbonne Universités, UPMC Univ Paris 06, CNRS UMR 7144, Adaptation et Diversité en Milieu Marin, Equipe ABICE, Station Biologique de Roscoff, 29680 Roscoff, France 2Present address: Laboratory of Biotechnology and Aquatic Genomics, Interdisciplinary Center for Aquaculture Research (INCAR), University of Concepcion, Concepción, Chile Running title: Bathymodiolus azoricus’s PGRPs and regulation of the symbiosis * Corresponding author: Dr. -

Immune Protein Orchestrates Daily Rhythm of Squid-Bacteria Symbiotic Relationship 19 October 2020, by Marcie Grabowski
Immune protein orchestrates daily rhythm of squid-bacteria symbiotic relationship 19 October 2020, by Marcie Grabowski National Academy of Sciences, could provide important clues on factors affecting human microbiome rhythms, as the MIF protein is also found in abundance in mammalian symbiotic tissues. To survive, the nocturnal Hawaiian bobtail squid depends on V. fischeri, which gives it the ability to mimic moonlight on the surface of the ocean and deceive monk seals and other predators, as it forages for food. The symbiotic bacteria also require nutrition, especially at night when they are more numerous and their light is required for the squid's camouflage. The research team, led by Eric Koch, who was a graduate researcher at the Pacific Biosciences Research Center (PBRC) in the UH Manoa School of Ocean and Earth Science and Technology (SOEST) at the time of the study, determined the squid regulates production of MIF as a way to A 63x magnification image showing the 4-week-old light control the movement of specialized immune cells, organ during the day showing EsMIF (magenta) called hemocytes, which provide chitin for bacteria surrounding the crypt spaces. Host DNA is labeled in to feed on. blue and the bacteria are labeled in green. Credit: Eric Koch At night, when the team found MIF was low in the squid's light organ, hemocytes were allowed into the regions where the bacteria reside and chitin was delivered. During the day, MIF was very high, Nearly every organism hosts a collection of which inhibits the hemocytes from coming into the symbiotic microbes—a microbiome. -

Squid Dissection
SQUID DISSECTION FOR THE TEACHER Discipline Biological Science Theme Scale and Structure Key Concept The investigation of the structure and function of an open ocean animal like the squid can be used to study adaptations for a completely pelagic existence. Synopsis Students work in pairs to dissect a squid and investigate its structure and how all the parts function together to allow the squid to survive and thrive in its open ocean environment. The squid is then honored as the students participate in a Squid Feast. Science Process Skills observing, communicating, comparing, classifying, relating Social Skill Checking for Understanding Vocabulary benthic, cephalopod, invertebrate, mollusc, pelagic, planktonic MATERIALS For INTO the Activities: Monterey Bay Aquarium Video Collection, "Seasons of the Squid" segment (Available for check-out from the MARE library or for purchase from Monterey Bay Aquarium) Squid Dissection 82 ©2001 The Regents of the University of California Anticipatory Chart #1 and #2 (see charts below) copied on large flip chart paper or on the board: Squid Dissection 83 ©2001 The Regents of the University of California For THROUGH the Activities: For each pair of students and one for yourself: One Squid. Available in grocery stores frozen in 3 lb. boxes for about $1 - $1.50/lb. There are about 32-35 squid per box or enough for two classes. Squid are also available fresh in fish markets. Frozen squid are the number one choice, but if only fresh are available, choose the largest and freshest ones and make sure they haven't been cleaned. Keep them in the freezer until the morning you are going to use them.