Comparative Morphology of Early Stages of Ommastrephid Squids from the Mediterranean Sea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Time of Day Affects Squid Catch in the U.S. Illex Illecebrosus Squid Fishery ∗ Eleanor A
Regional Studies in Marine Science 44 (2021) 101666 Contents lists available at ScienceDirect Regional Studies in Marine Science journal homepage: www.elsevier.com/locate/rsma Time of day affects squid catch in the U.S. Illex illecebrosus squid fishery ∗ Eleanor A. Bochenek a, , Eric N. Powell b a Haskin Shellfish Research Laboratory, Rutgers University, 6959 Miller Ave., Port Norris, NJ 08349, United States of America b Gulf Coast Research Laboratory, University of Southern Mississippi, 703 East Beach Dr, Ocean Springs, MS 39564, United States of America article info a b s t r a c t Article history: A mid-water otter trawl fishery targeting Illex illecebrosus operates in the northwestern Atlantic Ocean. Received 16 June 2020 The majority of I. illecebrosus are captured during mid-June to early September. Diel migratory behavior Received in revised form 28 January 2021 limits the fishery to daylight hours, but does the time-of-day affect catch? Illex illecebrosus were Accepted 8 February 2021 collected from each tow from a subset of the trawler fleet fishing on the outer continental shelf of Available online 10 February 2021 the Mid-Atlantic Bight over the fishing season to determine the influence of time-of-day on catch. The Keywords: male-to-female ratio was not influenced by time-of-day of capture. The size–frequency distribution of Illex illecebrosus I. illecebrosus varied between tows within the same day of capture. Time-of-day of capture influenced Short-fin squid average weight and, to a lesser degree, mantle length. Shorter and lighter squid were caught in the Time of day middle of the day. -

CEPHALOPODS 688 Cephalopods
click for previous page CEPHALOPODS 688 Cephalopods Introduction and GeneralINTRODUCTION Remarks AND GENERAL REMARKS by M.C. Dunning, M.D. Norman, and A.L. Reid iving cephalopods include nautiluses, bobtail and bottle squids, pygmy cuttlefishes, cuttlefishes, Lsquids, and octopuses. While they may not be as diverse a group as other molluscs or as the bony fishes in terms of number of species (about 600 cephalopod species described worldwide), they are very abundant and some reach large sizes. Hence they are of considerable ecological and commercial fisheries importance globally and in the Western Central Pacific. Remarks on MajorREMARKS Groups of CommercialON MAJOR Importance GROUPS OF COMMERCIAL IMPORTANCE Nautiluses (Family Nautilidae) Nautiluses are the only living cephalopods with an external shell throughout their life cycle. This shell is divided into chambers by a large number of septae and provides buoyancy to the animal. The animal is housed in the newest chamber. A muscular hood on the dorsal side helps close the aperture when the animal is withdrawn into the shell. Nautiluses have primitive eyes filled with seawater and without lenses. They have arms that are whip-like tentacles arranged in a double crown surrounding the mouth. Although they have no suckers on these arms, mucus associated with them is adherent. Nautiluses are restricted to deeper continental shelf and slope waters of the Indo-West Pacific and are caught by artisanal fishers using baited traps set on the bottom. The flesh is used for food and the shell for the souvenir trade. Specimens are also caught for live export for use in home aquaria and for research purposes. -
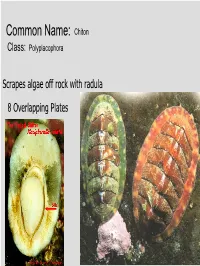
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Helminth Infection in the Short-Finned Squid Illex Coindetii (Cephalopoda, Ommastrephidae) Off NW Spain
DISEASES OF AQUATIC ORGANISMS Published September 14 Dis aquat Org Helminth infection in the short-finned squid Illex coindetii (Cephalopoda, Ommastrephidae) off NW Spain 'Laboratorio de Parasitologia, Facultad de Ciencias, Universidad de Vigo, Ap. 874 E-36200 Vigo, Spain 'Institute de Investigacions Marinas (CSIC),Eduardo Cabello 6, E-36208 Vigo, Spain ABSTRACT: A survey of parasites in 600 short-finned squid fllex coindetii (Verany. 1839) taken from 2 locations (north and south Galicia) off the northwestern Ibenan Peninsula revealed the presence of numerous somatoxenous helrninths. Three genera of Tetraphyllidean plerocercoids were represented (prevalences: Ph}~llobothriurn sp., 45.7%; Dinobothriunl sp., 0.8%; and Pelichnibothrium speciosum, 0.001 %); 1 Trypanorhynchidean metacestode was also present (Nybelinia vamagutll. 0.4 %). In addi- tion, larval nematodes of Anisakis simplex (L3) were recorded (10.6%). Abundance of infection was examined in relation to squid sex, standard length, maturity and locality. This analysis indicated that parasite infection was lower in the southern squids than in the northern squid group. Over the entire survey area, parasite infection showed a close positive correlation with host life-cycle, often with the greatest number of parasites among the largest and highest maturity individuals (>l8to 20 cm; matu- rlty stage V). KEY WORDS: Illex coindetii . Northwestern Iberian Peninsula Helminth parasites INTRODUCTION northeastern Atlantic waters. To this end, in the present paper some aspects of the host-parasite rela- Cephalopods represent 2.1 % of total worldwide tionship are examined. A possible local variability in catches of marine organisms (Guerra & Perez- degree of infection was also assessed in the light of the Gandaras 1983).In spite of the economic importance of clearly different hydrographical conditions between this fishery, relatively little is known about the host- northern and southern shelf areas off the Galician parasite relationships of teuthoid cephalopods (see coast (Fraga et al. -

The Cephalopoda
Carl Chun THE CEPHALOPO PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Carl Chun THE CEPHALOPODA NOTE TO PLATE LXVIII Figure 7 should read Figure 8 Figure 9 should read Figure 7 GERMAN DEEPSEA EXPEDITION 1898-1899. VOL. XVIII SCIENTIFIC RESULTS QF THE GERMAN DEEPSEA EXPEDITION ON BOARD THE*STEAMSHIP "VALDIVIA" 1898-1899 Volume Eighteen UNDER THE AUSPICES OF THE GERMAN MINISTRY OF THE INTERIOR Supervised by CARL CHUN, Director of the Expedition Professor of Zoology , Leipzig. After 1914 continued by AUGUST BRAUER Professor of Zoology, Berlin Carl Chun THE CEPHALOPODA PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Translatedfrom the German ISRAEL PROGRAM FOR SCIENTIFIC TRANSLATIONS Jerusalem 1975 TT 69-55057/2 Published Pursuant to an Agreement with THE SMITHSONIAN INSTITUTION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D.C. Since the study of the Cephalopoda is a very specialized field with a unique and specific terminology and phrase- ology, it was necessary to edit the translation in a technical sense to insure that as accurate and meaningful a represen- tation of Chun's original work as possible would be achieved. We hope to have accomplished this responsibility. Clyde F. E. Roper and Ingrid H. Roper Technical Editors Copyright © 1975 Keter Publishing House Jerusalem Ltd. IPST Cat. No. 05452 8 ISBN 7065 1260 X Translated by Albert Mercado Edited by Prof. O. Theodor Copy-edited by Ora Ashdit Composed, Printed and Bound by Keterpress Enterprises, Jerusalem, Israel Available from the U. S. DEPARTMENT OF COMMERCE National Technical Information Service Springfield, Va. 22151 List of Plates I Thaumatolampas diadema of luminous o.rgans 95 luminous organ 145 n.gen.n.sp. -

Planorbidae) from New Mexico
FRONT COVER—See Fig. 2B, p. 7. Circular 194 New Mexico Bureau of Mines & Mineral Resources A DIVISION OF NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY Pecosorbis, a new genus of fresh-water snails (Planorbidae) from New Mexico Dwight W. Taylor 98 Main St., #308, Tiburon, California 94920 SOCORRO 1985 iii Contents ABSTRACT 5 INTRODUCTION 5 MATERIALS AND METHODS 5 DESCRIPTION OF PECOSORBIS 5 PECOSORBIS. NEW GENUS 5 PECOSORBIS KANSASENSIS (Berry) 6 LOCALITIES AND MATERIAL EXAMINED 9 Habitat 12 CLASSIFICATION AND RELATIONSHIPS 12 DESCRIPTION OF MENETUS 14 GENUS MENETUS H. AND A. ADAMS 14 DESCRIPTION OF MENETUS CALLIOGLYPTUS 14 REFERENCES 17 Figures 1—Pecosorbis kansasensis, shell 6 2—Pecosorbis kansasensis, shell removed 7 3—Pecosorbis kansasensis, penial complex 8 4—Pecosorbis kansasensis, reproductive system 8 5—Pecosorbis kansasensis, penial complex 9 6—Pecosorbis kansasensis, ovotestis and seminal vesicle 10 7—Pecosorbis kansasensis, prostate 10 8—Pecosorbis kansasensis, penial complex 10 9—Pecosorbis kansaensis, composite diagram of penial complex 10 10—Pecosorbis kansasensis, distribution map 11 11—Menetus callioglyptus, reproductive system 15 12—Menetus callioglyptus, penial complex 15 13—Menetus callioglyptus, penial complex 16 14—Planorbella trivolvis lenta, reproductive system 16 Tables 1—Comparison of Menetus and Pecosorbis 13 5 Abstract Pecosorbis, new genus of Planorbidae, subfamily Planorbulinae, is established for Biomphalaria kansasensis Berry. The species has previously been known only as a Pliocene fossil, but now is recognized in the Quaternary of the southwest United States, and living in the Pecos Valley of New Mexico. Pecosorbis is unusual because of its restricted distribution and habitat in seasonal rock pools. Most similar to Menetus, it differs in having a preputial organ with an external duct, no spermatheca, and a penial sac that is mostly eversible. -

Spermatophore Transfer in Illex Coindetii (Cephalopoda: Ommastrephidae)
Spermatophore transfer in Illex coindetii (Cephalopoda: Ommastrephidae) TREBALL DE FI DE GRAU GRAU DE CIÈNCIES DEL MAR EVA DÍAZ ZAPATA Institut de Ciències del Mar (CSIC) Universitat de Barcelona Tutors: Fernando Ángel Fernández-Álvarez i Roger Villanueva 05, 2019 RESUMEN CIENTÍFICO La transmisión de esperma desde el macho a la hembra es un proceso crítico durante la reproducción que asegura la posterior fecundación de oocitos. Durante el apareamiento, los machos de los cefalópodos incrustan en el tejido de la hembra paquetes de esperma denominados espermatóforos mediante un complejo proceso de evaginación conocido como reacción espermatofórica. Estos reservorios de esperma incrustados en el cuerpo de la hembra se denominan espermatangios. En este estudio se han analizado machos y hembras maduros de Illex coindetii recolectados desde diciembre del 2018 hasta abril del 2019 en la lonja de pescadores de Vilanova i la Geltrú (Mediterráneo NO). El objetivo de este estudio es entender cómo se produce la transmisión de los espermatóforos en esta especie carente de órganos especiales para el almacenamiento de esperma (receptáculos seminales). En los ejemplares estudiados se cuantificó el número de espermatóforos y espermatangios y mediante experimentos in vitro se indujo la reacción espermatofórica para describir el proceso de liberación del esperma. Los resultados han demostrado que los machos maduros disponen entre 143 y 1654 espermatóforos y las hembras copuladas presentan entre 35 y 668 espermatangios en su interior. La inversión reproductiva en cada cópula realizada por los machos oscila entre el 2 y el 40 % del número de espermatóforos disponibles en un momento dado. En experimentos realizados in vitro, la reacción espermatofórica se inicia espontáneamente tras entrar el espermatóforo en contacto con el agua de mar. -

Husbandry Manual for BLUE-RINGED OCTOPUS Hapalochlaena Lunulata (Mollusca: Octopodidae)
Husbandry Manual for BLUE-RINGED OCTOPUS Hapalochlaena lunulata (Mollusca: Octopodidae) Date By From Version 2005 Leanne Hayter Ultimo TAFE v 1 T A B L E O F C O N T E N T S 1 PREFACE ................................................................................................................................ 5 2 INTRODUCTION ...................................................................................................................... 6 2.1 CLASSIFICATION .............................................................................................................................. 8 2.2 GENERAL FEATURES ....................................................................................................................... 8 2.3 HISTORY IN CAPTIVITY ..................................................................................................................... 9 2.4 EDUCATION ..................................................................................................................................... 9 2.5 CONSERVATION & RESEARCH ........................................................................................................ 10 3 TAXONOMY ............................................................................................................................12 3.1 NOMENCLATURE ........................................................................................................................... 12 3.2 OTHER SPECIES ........................................................................................................................... -

Giant Pacific Octopus (Enteroctopus Dofleini) Care Manual
Giant Pacific Octopus Insert Photo within this space (Enteroctopus dofleini) Care Manual CREATED BY AZA Aquatic Invertebrate Taxonomic Advisory Group IN ASSOCIATION WITH AZA Animal Welfare Committee Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Aquatic Invertebrate Taxon Advisory Group (AITAG) (2014). Giant Pacific Octopus (Enteroctopus dofleini) Care Manual. Association of Zoos and Aquariums, Silver Spring, MD. Original Completion Date: September 2014 Dedication: This work is dedicated to the memory of Roland C. Anderson, who passed away suddenly before its completion. No one person is more responsible for advancing and elevating the state of husbandry of this species, and we hope his lifelong body of work will inspire the next generation of aquarists towards the same ideals. Authors and Significant Contributors: Barrett L. Christie, The Dallas Zoo and Children’s Aquarium at Fair Park, AITAG Steering Committee Alan Peters, Smithsonian Institution, National Zoological Park, AITAG Steering Committee Gregory J. Barord, City University of New York, AITAG Advisor Mark J. Rehling, Cleveland Metroparks Zoo Roland C. Anderson, PhD Reviewers: Mike Brittsan, Columbus Zoo and Aquarium Paula Carlson, Dallas World Aquarium Marie Collins, Sea Life Aquarium Carlsbad David DeNardo, New York Aquarium Joshua Frey Sr., Downtown Aquarium Houston Jay Hemdal, Toledo -

Vertical Distribution of Pelagic Cephalopods *
* Vertical Distribution of Pelagic Cephalopods CLYDE F. E. ROPER and RICHARD E. YOUNG SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 209 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Insti- tution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. These pub- lications are distributed by mailing lists to libraries, laboratories, and other interested institutions and specialists throughout the world. Individual copies may be obtained from the Smithsonian Institution Press as long as stocks are available. S. DILLON RIPLEY Secretary Smithsonian Institution SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 209 Vertical Distribution of Pelagic Cephalopds Clyde F. -

Monda Y , March 22, 2021
NATIONAL SHELLFISHERIES ASSOCIATION Program and Abstracts of the 113th Annual Meeting March 22 − 25, 2021 Global Edition @ http://shellfish21.com Follow on Social Media: #shellfish21 NSA 113th ANNUAL MEETING (virtual) National Shellfisheries Association March 22—March 25, 2021 MONDAY, MARCH 22, 2021 DAILY MEETING UPDATE (LIVE) 8:00 AM Gulf of Maine Gulf of Maine Gulf of Mexico Puget Sound Chesapeake Bay Monterey Bay SHELLFISH ONE HEALTH: SHELLFISH AQUACULTURE EPIGENOMES & 8:30-10:30 AM CEPHALOPODS OYSTER I RESTORATION & BUSINESS & MICROBIOMES: FROM SOIL CONSERVATION ECONOMICS TO PEOPLE WORKSHOP 10:30-10:45 AM MORNING BREAK THE SEA GRANT SHELLFISH ONE HEALTH: EPIGENOMES COVID-19 RESPONSE GENERAL 10:45-1:00 PM OYSTER I RESTORATION & & MICROBIOMES: FROM SOIL TO THE NEEDS OF THE CONTRIBUTED I CONSERVATION TO PEOPLE WORKSHOP SHELLFISH INDUSTRY 1:00-1:30 PM LUNCH BREAK WITH SPONSOR & TRADESHOW PRESENTATIONS PLENARY LECTURE: Roger Mann (Virginia Institute of Marine Science, USA) (LIVE) 1:30-2:30 PM Chesapeake Bay EASTERN OYSTER SHELLFISH ONE HEALTH: EPIGENOMES 2:30-3:45 PM GENOME CONSORTIUM BLUE CRABS VIBRIO RESTORATION & & MICROBIOMES: FROM SOIL WORKSHOP CONSERVATION TO PEOPLE WORKSHOP BLUE CRAB GENOMICS EASTERN OYSTER & TRANSCRIPTOMICS: SHELLFISH ONE HEALTH: EPIGENOMES 3:45–5:45 PM GENOME CONSORTIUM THE PROGRAM OF THE VIBRIO RESTORATION & & MICROBIOMES: FROM SOIL WORKSHOP BLUE CRAB GENOME CONSERVATION TO PEOPLE WORKSHOP PROJECT TUESDAY, MARCH 23, 2021 DAILY MEETING UPDATE (LIVE) 8:00 AM Gulf of Maine Gulf of Maine Gulf of Mexico Puget Sound -

Marine Ecology Progress Series 514:105
Vol. 514: 105–118, 2014 MARINE ECOLOGY PROGRESS SERIES Published November 6 doi: 10.3354/meps10972 Mar Ecol Prog Ser Role of hydro-climatic and demographic processes on the spatio-temporal distribution of cephalopods in the western Mediterranean Patricia Puerta1,*, Manuel Hidalgo1, María González2, Antonio Esteban3, Antoni Quetglas1 1Instituto Español de Oceanografía, Centre Oceanogràfic de les Balears, Moll de Ponent s/n, Apdo. 291, 07015 Palma de Mallorca, Spain 2Instituto Español de Oceanografía, Centro Oceanográfico de Málaga, Puerto Pesquero s/n, 29640 Fuengirola, Málaga, Spain 3Instituto Español de Oceanografía, Centro Oceanográfico de Murcia, Varadero 1, 30740 San Pedro del Pinatar, Murcia, Spain ABSTRACT: Fluctuations in marine populations occur both in terms of abundance and distribu- tional ranges, and this has major implications for marine ecology and fisheries management. How- ever, little is known about the variability in, and factors influencing, spatial dynamics in marine groups other than fish. Using time series data from trawl surveys conducted in the western Medi- terranean Sea from 1994 to 2012, we analysed the variability in population distribution (latitude, longitude and depth) of 2 cephalopod species with contrasting life histories, viz. the nektobenthic squid Illex coindetii and the benthic octopus Eledone cirrhosa. We investigated the influence of demographic information (population density and mean individual size) together with environ- mental variables (including chlorophyll a concentration, runoff, precipitation, temperature and climate) to identify the main drivers shaping the cephalopod distributions in 4 shelf regions with contrasting oceanographic and geographic conditions. Marked inter-annual fluctuations were found in the distribution of the 2 species. In general, squid and octopus populations were affected by the same variables, but the effect of each variable depended on the species and study region.