Biochemistry and Structure-Function Relationships in the Proteinaceous Egg Capsules of Busycotypus Canaliculatus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CEPHALOPODS 688 Cephalopods
click for previous page CEPHALOPODS 688 Cephalopods Introduction and GeneralINTRODUCTION Remarks AND GENERAL REMARKS by M.C. Dunning, M.D. Norman, and A.L. Reid iving cephalopods include nautiluses, bobtail and bottle squids, pygmy cuttlefishes, cuttlefishes, Lsquids, and octopuses. While they may not be as diverse a group as other molluscs or as the bony fishes in terms of number of species (about 600 cephalopod species described worldwide), they are very abundant and some reach large sizes. Hence they are of considerable ecological and commercial fisheries importance globally and in the Western Central Pacific. Remarks on MajorREMARKS Groups of CommercialON MAJOR Importance GROUPS OF COMMERCIAL IMPORTANCE Nautiluses (Family Nautilidae) Nautiluses are the only living cephalopods with an external shell throughout their life cycle. This shell is divided into chambers by a large number of septae and provides buoyancy to the animal. The animal is housed in the newest chamber. A muscular hood on the dorsal side helps close the aperture when the animal is withdrawn into the shell. Nautiluses have primitive eyes filled with seawater and without lenses. They have arms that are whip-like tentacles arranged in a double crown surrounding the mouth. Although they have no suckers on these arms, mucus associated with them is adherent. Nautiluses are restricted to deeper continental shelf and slope waters of the Indo-West Pacific and are caught by artisanal fishers using baited traps set on the bottom. The flesh is used for food and the shell for the souvenir trade. Specimens are also caught for live export for use in home aquaria and for research purposes. -
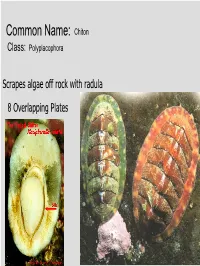
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

The Cephalopoda
Carl Chun THE CEPHALOPO PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Carl Chun THE CEPHALOPODA NOTE TO PLATE LXVIII Figure 7 should read Figure 8 Figure 9 should read Figure 7 GERMAN DEEPSEA EXPEDITION 1898-1899. VOL. XVIII SCIENTIFIC RESULTS QF THE GERMAN DEEPSEA EXPEDITION ON BOARD THE*STEAMSHIP "VALDIVIA" 1898-1899 Volume Eighteen UNDER THE AUSPICES OF THE GERMAN MINISTRY OF THE INTERIOR Supervised by CARL CHUN, Director of the Expedition Professor of Zoology , Leipzig. After 1914 continued by AUGUST BRAUER Professor of Zoology, Berlin Carl Chun THE CEPHALOPODA PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Translatedfrom the German ISRAEL PROGRAM FOR SCIENTIFIC TRANSLATIONS Jerusalem 1975 TT 69-55057/2 Published Pursuant to an Agreement with THE SMITHSONIAN INSTITUTION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D.C. Since the study of the Cephalopoda is a very specialized field with a unique and specific terminology and phrase- ology, it was necessary to edit the translation in a technical sense to insure that as accurate and meaningful a represen- tation of Chun's original work as possible would be achieved. We hope to have accomplished this responsibility. Clyde F. E. Roper and Ingrid H. Roper Technical Editors Copyright © 1975 Keter Publishing House Jerusalem Ltd. IPST Cat. No. 05452 8 ISBN 7065 1260 X Translated by Albert Mercado Edited by Prof. O. Theodor Copy-edited by Ora Ashdit Composed, Printed and Bound by Keterpress Enterprises, Jerusalem, Israel Available from the U. S. DEPARTMENT OF COMMERCE National Technical Information Service Springfield, Va. 22151 List of Plates I Thaumatolampas diadema of luminous o.rgans 95 luminous organ 145 n.gen.n.sp. -

Planorbidae) from New Mexico
FRONT COVER—See Fig. 2B, p. 7. Circular 194 New Mexico Bureau of Mines & Mineral Resources A DIVISION OF NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY Pecosorbis, a new genus of fresh-water snails (Planorbidae) from New Mexico Dwight W. Taylor 98 Main St., #308, Tiburon, California 94920 SOCORRO 1985 iii Contents ABSTRACT 5 INTRODUCTION 5 MATERIALS AND METHODS 5 DESCRIPTION OF PECOSORBIS 5 PECOSORBIS. NEW GENUS 5 PECOSORBIS KANSASENSIS (Berry) 6 LOCALITIES AND MATERIAL EXAMINED 9 Habitat 12 CLASSIFICATION AND RELATIONSHIPS 12 DESCRIPTION OF MENETUS 14 GENUS MENETUS H. AND A. ADAMS 14 DESCRIPTION OF MENETUS CALLIOGLYPTUS 14 REFERENCES 17 Figures 1—Pecosorbis kansasensis, shell 6 2—Pecosorbis kansasensis, shell removed 7 3—Pecosorbis kansasensis, penial complex 8 4—Pecosorbis kansasensis, reproductive system 8 5—Pecosorbis kansasensis, penial complex 9 6—Pecosorbis kansasensis, ovotestis and seminal vesicle 10 7—Pecosorbis kansasensis, prostate 10 8—Pecosorbis kansasensis, penial complex 10 9—Pecosorbis kansaensis, composite diagram of penial complex 10 10—Pecosorbis kansasensis, distribution map 11 11—Menetus callioglyptus, reproductive system 15 12—Menetus callioglyptus, penial complex 15 13—Menetus callioglyptus, penial complex 16 14—Planorbella trivolvis lenta, reproductive system 16 Tables 1—Comparison of Menetus and Pecosorbis 13 5 Abstract Pecosorbis, new genus of Planorbidae, subfamily Planorbulinae, is established for Biomphalaria kansasensis Berry. The species has previously been known only as a Pliocene fossil, but now is recognized in the Quaternary of the southwest United States, and living in the Pecos Valley of New Mexico. Pecosorbis is unusual because of its restricted distribution and habitat in seasonal rock pools. Most similar to Menetus, it differs in having a preputial organ with an external duct, no spermatheca, and a penial sac that is mostly eversible. -

Biological Materials: a Materials Science Approach✩
JOURNALOFTHEMECHANICALBEHAVIOROFBIOMEDICALMATERIALS ( ) ± available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jmbbm Review article Biological materials: A materials science approachI Marc A. Meyers∗, PoYu Chen, Maria I. Lopez, Yasuaki Seki, Albert Y.M. Lin University of California, San Diego, La Jolla, CA, United States ARTICLEINFO ABSTRACT Article history: The approach used by Materials Science and Engineering is revealing new aspects Received 25 May 2010 in the structure and properties of biological materials. The integration of advanced Received in revised form characterization, mechanical testing, and modeling methods can rationalize heretofore 20 August 2010 unexplained aspects of these structures. As an illustration of the power of this Accepted 22 August 2010 methodology, we apply it to biomineralized shells, avian beaks and feathers, and fish scales. We also present a few selected bioinspired applications: Velcro, an Al2O3PMMA composite inspired by the abalone shell, and synthetic attachment devices inspired by gecko. ⃝c 2010 Elsevier Ltd. All rights reserved. Contents 1. Introduction and basic components ............................................................................................................................................. 1 2. Hierarchical nature of biological materials ................................................................................................................................... 3 3. Structural biological materials..................................................................................................................................................... -

Monda Y , March 22, 2021
NATIONAL SHELLFISHERIES ASSOCIATION Program and Abstracts of the 113th Annual Meeting March 22 − 25, 2021 Global Edition @ http://shellfish21.com Follow on Social Media: #shellfish21 NSA 113th ANNUAL MEETING (virtual) National Shellfisheries Association March 22—March 25, 2021 MONDAY, MARCH 22, 2021 DAILY MEETING UPDATE (LIVE) 8:00 AM Gulf of Maine Gulf of Maine Gulf of Mexico Puget Sound Chesapeake Bay Monterey Bay SHELLFISH ONE HEALTH: SHELLFISH AQUACULTURE EPIGENOMES & 8:30-10:30 AM CEPHALOPODS OYSTER I RESTORATION & BUSINESS & MICROBIOMES: FROM SOIL CONSERVATION ECONOMICS TO PEOPLE WORKSHOP 10:30-10:45 AM MORNING BREAK THE SEA GRANT SHELLFISH ONE HEALTH: EPIGENOMES COVID-19 RESPONSE GENERAL 10:45-1:00 PM OYSTER I RESTORATION & & MICROBIOMES: FROM SOIL TO THE NEEDS OF THE CONTRIBUTED I CONSERVATION TO PEOPLE WORKSHOP SHELLFISH INDUSTRY 1:00-1:30 PM LUNCH BREAK WITH SPONSOR & TRADESHOW PRESENTATIONS PLENARY LECTURE: Roger Mann (Virginia Institute of Marine Science, USA) (LIVE) 1:30-2:30 PM Chesapeake Bay EASTERN OYSTER SHELLFISH ONE HEALTH: EPIGENOMES 2:30-3:45 PM GENOME CONSORTIUM BLUE CRABS VIBRIO RESTORATION & & MICROBIOMES: FROM SOIL WORKSHOP CONSERVATION TO PEOPLE WORKSHOP BLUE CRAB GENOMICS EASTERN OYSTER & TRANSCRIPTOMICS: SHELLFISH ONE HEALTH: EPIGENOMES 3:45–5:45 PM GENOME CONSORTIUM THE PROGRAM OF THE VIBRIO RESTORATION & & MICROBIOMES: FROM SOIL WORKSHOP BLUE CRAB GENOME CONSERVATION TO PEOPLE WORKSHOP PROJECT TUESDAY, MARCH 23, 2021 DAILY MEETING UPDATE (LIVE) 8:00 AM Gulf of Maine Gulf of Maine Gulf of Mexico Puget Sound -

Online Dictionary of Invertebrate Zoology: A
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Armand R. Maggenti Online Dictionary of Invertebrate Zoology Parasitology, Harold W. Manter Laboratory of September 2005 Online Dictionary of Invertebrate Zoology: A Mary Ann Basinger Maggenti University of California-Davis Armand R. Maggenti University of California, Davis Scott Lyell Gardner University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/onlinedictinvertzoology Part of the Zoology Commons Maggenti, Mary Ann Basinger; Maggenti, Armand R.; and Gardner, Scott Lyell, "Online Dictionary of Invertebrate Zoology: A" (2005). Armand R. Maggenti Online Dictionary of Invertebrate Zoology. 16. https://digitalcommons.unl.edu/onlinedictinvertzoology/16 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Armand R. Maggenti Online Dictionary of Invertebrate Zoology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Online Dictionary of Invertebrate Zoology 2 abdominal filament see cercus A abdominal ganglia (ARTHRO) Ganglia of the ventral nerve cord that innervate the abdomen, each giving off a pair of principal nerves to the muscles of the segment; located between the alimentary canal and the large ventral mus- cles. abactinal a. [L. ab, from; Gr. aktis, ray] (ECHINOD) Of or per- taining to the area of the body without tube feet that nor- abdominal process (ARTHRO: Crustacea) In Branchiopoda, mally does not include the madreporite; not situated on the fingerlike projections on the dorsal surface of the abdomen. ambulacral area; abambulacral. abactinally adv. abdominal somite (ARTHRO: Crustacea) Any single division of abambulacral see abactinal the body between the thorax and telson; a pleomere; a pleonite. -

In Comparison with Lymnaea Stagnalis (Linnaeus, 1758) K
Anatomical and histological characterization of the gametogenesis of Radix balthica (linnaeus, 1758) in comparison with Lymnaea stagnalis (linnaeus, 1758) K. Abbaci, S. Joachirn, J. Garric, P. Boisseaux, J.M. Exbrayat, J.M. Porcher, O. Geffard To cite this version: K. Abbaci, S. Joachirn, J. Garric, P. Boisseaux, J.M. Exbrayat, et al.. Anatomical and histological characterization of the gametogenesis of Radix balthica (linnaeus, 1758) in comparison with Lymnaea stagnalis (linnaeus, 1758). Journal of Histology Histopathology, 2017, 4, pp.5. <10.7243/2055-091X-4-5>. <hal-01734875> HAL Id: hal-01734875 https://hal.archives-ouvertes.fr/hal-01734875 Submitted on 15 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Journal of Histology & Histopathology ISSN 2055-091X | Volume 4 | Article 5 Special Section | Histology of normal tissues | Original Research Open Access Anatomical and histological characterization of the gametogenesis of Radix balthica (linnaeus, 1758) in comparison with Lymnaea stagnalis (linnaeus, 1758) Khedidja Tair-Abbaci1*, Sandrine Joachim2, Jeanne Garric1, Paul Boisseaux1, Jean-Marie Exbrayat3, Jean-Marc Porcher2 and Olivier Geffard1 *Correspondence: [email protected] CrossMark ← Click for updates 1Irstea, UR MALY, Lyon-Villeurbanne center, 5 rue de la Doua, BP 32108, 69616 Villeurbanne Cedex, France. -

Calma Glaucoides: a Study in Adaptation
Calma Glaucoides: A study in adaptation. By T. J. Evans, M.A. (Oxoii.), Lecturer in the Medical School, Guy's Hospital, London University. With Plate 11 and 3 Text-figures. A' DETAILED description of certain portions of the anatomy of a small British mollusc is here submitted, not so much as an extension of our knowledge of molluscan structure, as on account of the general biological interest of a unique metabolic type. Whilst retaining the shape and general structural plan of an ueolidiomorph Nudibranch, Calma presents a combination of important departures from that type which may all be directly or indirectly referred to its specialized diet, namely, the eggs and embryos of the smaller shore fishes. So close is the external resemblance to the Aeolid that Alder and Hancock originally (1, PI. xxii, letterpress) placed it in Cuvier's genus Eolis, whereas the modifications to be described are in some respects so great as to be comparable with those associated with a parasitic life. The genus has been recorded only from European waters, and contains Calma glaucoides of Alder and Hancock, commonly taken at Plymouth, Roscoff, and Concarneau, the Eolis albicans of Friele and Hansen (5) from the North Atlantic, and the Forestia mirabilis of Trinchese (9) from the Mediterranean. All three will probably be found on re-examination to belong to one species, C. glaucoides. At Roscoff, Hecht (6) found the animal feeding during June and July on developing eggs of Cottus, Lepadogaster and Liparis under stones, and in September in the swollen radical 440 T. J. EVANS sacs of Laminaria flexicaulis. -

Comparative Morphology of Early Stages of Ommastrephid Squids from the Mediterranean Sea
INTERUNIVERSITY MASTER OF AQUACULTURE 2011 – 2012 Comparative morphology of early stages of ommastrephid squids from the Mediterranean Sea Student GIULIANO PETRONI Tutor Dra. MERCÈ DURFORT Department of Cellular Biology , Faculty of Biology, University of Barcelona Principal Investigator Dr. ROGER VILLANUEVA (PI) Depart ment of renewable marine resources, Institut de Ciències del Mar, CSIC Research Institute Institut de Ciències del Mar, CSIC, Barcelona ABSTRACT Early life of oceanic squids is poorly known due to the difficulties in locating their pelagic egg masses in the wild or obtaining them under laboratory conditions. Recent in vitro fertilization techniques were used in this study to provide first comparative data of the early stages of the most important ommastrephid squid species from the Mediterranean Sea: Illex coindetii , Todaropsis eblanae and Todarodes sagittatus . Eggs, embryos and newly hatched paralarvae were described through development highlighting sizes and morphological differences between species. Duration of embryonic development in I. coindetii and T. eblanae was strictly correlated with temperature and egg size. Embryos of T. sagittatus were unable to reach hatchling stage and died during organogenesis. With the aim to distinguish rhynchoutheuthion larvae of I. coindetii and T. eblanae , particular attention was given to a few types of characters useful for species identification. The general structure of arm and proboscis suckers was described based on the presence of knobs on the chitinous ring. Chromatophore patterns on mantle and head were given for hatchlings of both species and showed some individual variation. A peculiar skin sculpture was observed under a binocular microscope on the external mantle surface of T. eblanae . SEM analysis revealed the presence of a network of hexagonal cells covered by dermal structures which may have a high taxonomic value. -

Alexander 2013 Principles-Of-Animal-Locomotion.Pdf
.................................................... Principles of Animal Locomotion Principles of Animal Locomotion ..................................................... R. McNeill Alexander PRINCETON UNIVERSITY PRESS PRINCETON AND OXFORD Copyright © 2003 by Princeton University Press Published by Princeton University Press, 41 William Street, Princeton, New Jersey 08540 In the United Kingdom: Princeton University Press, 3 Market Place, Woodstock, Oxfordshire OX20 1SY All Rights Reserved Second printing, and first paperback printing, 2006 Paperback ISBN-13: 978-0-691-12634-0 Paperback ISBN-10: 0-691-12634-8 The Library of Congress has cataloged the cloth edition of this book as follows Alexander, R. McNeill. Principles of animal locomotion / R. McNeill Alexander. p. cm. Includes bibliographical references (p. ). ISBN 0-691-08678-8 (alk. paper) 1. Animal locomotion. I. Title. QP301.A2963 2002 591.47′9—dc21 2002016904 British Library Cataloging-in-Publication Data is available This book has been composed in Galliard and Bulmer Printed on acid-free paper. ∞ pup.princeton.edu Printed in the United States of America 1098765432 Contents ............................................................... PREFACE ix Chapter 1. The Best Way to Travel 1 1.1. Fitness 1 1.2. Speed 2 1.3. Acceleration and Maneuverability 2 1.4. Endurance 4 1.5. Economy of Energy 7 1.6. Stability 8 1.7. Compromises 9 1.8. Constraints 9 1.9. Optimization Theory 10 1.10. Gaits 12 Chapter 2. Muscle, the Motor 15 2.1. How Muscles Exert Force 15 2.2. Shortening and Lengthening Muscle 22 2.3. Power Output of Muscles 26 2.4. Pennation Patterns and Moment Arms 28 2.5. Power Consumption 31 2.6. Some Other Types of Muscle 34 Chapter 3. -

Elastomeric Proteins: Structures, Biomechanical Properties, and Biological Roles Edited by Peter R
Cambridge University Press 0521815940 - Elastomeric Proteins: Structures, Biomechanical Properties, and Biological Roles Edited by Peter R. Shewry, Arthur S. Tatham and Allen J. Bailey Index More information Index abalone, 353–54 8-anilinonaphthalene sulfonate (ANS), AB-block copolymers, 303, 307, 310–11, 271 313–14 anisotropic elastomers, 302. See also liquid abductin, 322, 323, 339–40, 344, 346 crystal elastomers ABP280/filamin 1, 233 ankyrins, 215–17, 222, 235 aciniform glands, 155 Antherea, 198 acoustic absorption, 69–73 antifibrillin-1, 102–3 actins, 215–16, 226–28, 230–31, 233, 345 ants, 265 adhesives, 162–63, 355, 359–60 aorta, elastin function in, 11 Aedes aegypti, 270 apical contractile rings, 232 Aeshna grandis, 259 apodeme, 2 alanine, 205, 272 aponeurosis, 6 Alexander, R. McNeill, 1–14, 27 Araneus, 155. See also spider silks allysine aldol, 43 Araneus diadematus ␣-actinin, 213–14 functional design of silks, 33–34, 36 ␣-catenin, 215–16 glycoprotein glue, 162–63 ␣-elastin, 62–63, 75–77 material properties of silk, 19 amino acid sequences, 338–42 protein sequences of silk, 142 in abductin, 339–40 spiral vs. radius silk properties, in elastin, 340 158–63 in fibrillins, 342 typical orb web of, 154, 157–58 in gluten, 283–90, 340–41 water as plasticizer, 160–64 in mussel byssus, 199–200, 340 Araneus gemmoides, 138, 144–45 in resilin, 261–63, 266–67, 340 Araneus sericatus,33 in spectrins, 342 Argopectin, 339 in spider silks, 138–44, 147, 155–56, 313, Argyroneta aquatica, 153 340 arteries, elastin function in, 11, 20 in titin, 340, 342 asparagine, 268 ampullate gland.